Case Report

Congenital Aggressive Angiomyxoma of the Vulva: A Rarity
*Nidhi Mahajan, *Mitali Agarwal, * Chabbi Ranu Gupta,
- * Department of Pathology & Dept of Pediatric Surgery, Chacha Nehru Bal Chikitsalaya Geeta Colony, Delhi
- Submitted: Tuesday, August 7, 2018
- Accepted:Friday, September 7, 2018
- Published:Saturday, September 8, 2018
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ((http://creativecommons.org/licenses/by/4.0)which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background:
Aggressive angiomyxoma (AA) is an uncommon locally invasive mesenchymal tumor in females of reproductive age group. Rarely, seen in young children and clinically misdiagnosed because of nonspecific radiological features, low cellularity on FNAC and superficial location, less amenable to trucut biopsy.
Case:
We report a 7 year old girl who presented with a swelling in the left labia majora, since birth. It was excised and was diagnosed as an aggressive angiomyxoma on histopathology.
Conclusion:
AA is an important differential in cases of slow growing vulvar masses in children. Histopathology and immunohistochemistry can differentiate its morphological mimics. Long term surveillance is needed in view of high recurrence rates.
Introduction
Aggressive angiomyxoma (AA) is an uncommon local mesenchymal tumor of poorly known etiology usually affecting vulva, perineal region, buttocks or pelvis of women of reproductive age group (mid 40s) [1]. The lesion is characterized by an insidious and locally infiltrating growth pattern however is non metastasising. The tumour is known for its recurrence, so much so that despite several treatment modalities, relapse rate is as high as 72% [2]. This case is being reported for its rarity and congenital presentation of a superficial aggressive angiomyxoma of vulva in a 7 year old girl. It is emphasised that this entity should also be kept in mind while considering differentials of vulvar lesions in pediatric population.
Case
A 7 year old girl presented with a swelling in the left labia majora, present since birth and slowly growing. There was a recent onset of sudden increase in size which brought the patient to the surgical OPD. No other relevant medical history could be elicited. On local examination, swelling was 3 x 2.5 cms, soft to firm, mildly tender and showed restricted mobility (Figure 1a). There was no inguinal lymphadenopathy. Fine needle aspiration was not performed. Ultrasound yielded a heterogeneous hyperechoic mass with peripheral vascularity and no mitotic activity. With the clinical impression of a lipoma or fibroepithelial polyp, the mass was excised and sent for histopathological examination. We received a skin covered nodular soft tissue measuring 2 x 2 x 1.5 cms in size. Cut section was grey white, homogenous glistening with few congested areas. Microscopy revealed a well circumscribed, hypocellular lesion comprising of spindled and stellate shaped cells dispersed in a myxoid background (Figure 1b). Also seen were thin walled variable sized vascular channels (Figure 1c). There were few interspersed and scattered neutrophils, lymphocytes and muciphages. No mitosis, nuclear pleomorphism or necrosis was identified. The lesion was seen extending in deeper soft tissue, though resected margin was free. On immunohistochemistry, the tumor cells were positive for Vimentin and CD 34 (Figure 1d). A final diagnosis of an aggressive angiomyxoma was made. The patient was kept in follow up and has not developed any recurrence till date.
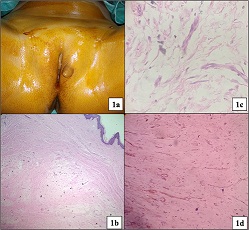

Figure 1: 1a- Pedunculated polypoidal mass at labia. 1b- Photomicrograph shows a well circumscribed, hypocellular lesion comprising of stellate shaped cells dispersed in a myxoid stroma (Haematoxylin & Eosin, 40X). 1c- High power view of the interspersed thin, vascular channels (Haematoxylin & Eosin, 400X). 1d- Endothelial cells showing CD34 positivity on IHC (Immunohistochemistry, DAB as chromogen).
Discussion
Angiomyxomas were first described in literature by Steeper and Rosai in 1983 [3], since then very few case reports have been reported in children < 14 years of age. They are classified as two types: superficial and aggressive. The former are usually seen in the head and neck, preauricular region, lower eyelid and alveolus. The latter is characterised by its propensity for local invasion and high rate of recurrence post excision. It shows predisposition for females of reproductive age group and usually involves perineal pelvic region. Males are less commonly affected and show involvement of scrotum.
The pathogenesis of the tumor is still debatable. The tumor is believed to arise from specialized mesenchymal cells of the pelvic–perineal region or from the multipotent perivascular progenitor cells, which display variable myofibroblastic and fibroblastic features. This hypothesis is supported by the fact that the tumor cells express desmin and in some cases, a smooth muscle actin along with desmin. Interesting molecular studies have linked a consistent clonal aberration of the chromosome 12, in the region 12q13– 15, associated with rearrangement of the HMGIC gene (highmobility group protein isoform I-C) with AA. The study claims that AA is molecularly part of the benign group of mesenchymal tumours showing multiple aberration region involvement. HMGIC expression in AA is of value in the histological differential diagnosis. Detection of inappropriate HMGI-C expression using the immunoperoxidase technique with anti HMGI-C antibody is of proven value in histological differential diagnosis and also may potentially be a useful marker for microscopic residual disease.
Clinically, these lesions present as an insidiously growing, polypoidal cutaneous mass, often misdiagnosed as Gartner’s, Bartholin’s cyst, simple labial cyst, lipomas, vaginal polyps, fibromas, neural tumors and pelvic floor hernias more so in the young patients as AA is a commoner entity in adults. On histopathology, gross examination of AA reveals a soft, well defined mass ranging in size from few centimetres to 20 cms or even more. Cut section, is homogenous and glistening in appearance. Microscopically, the tumour is composed of widely scattered spindled to stellate-shaped cells with moderate cytoplasm and small round to oval hyperchromatic nuclei with inconspicuous nucleoli, embedded in a myxoid stroma. The defining feature is the presence of variable sized vessels that range from small thin-walled capillaries to large vessels with secondary changes like perivascular hyalinization and medial hypertrophy. Immunohistochemically, most AA express different combinations of vimentin, desmin, smooth-muscle actin, muscle-specific actin, CD34, and CD44, but all are invariably negative for S-100, CEA, and keratin (these being neural and epithelial markers respectively). The tumor shows consistent expression of Estrogen and Progesterone receptors for both genders, suggesting a connection with hormones on growth of these lesions.
There is a wide differential diagnosis of AA including both benign and low grade malignant myxoid lesions like cellular angiofibromas, myxoid variants of neurothekomas, neurofibromas, superficial myofibroblastoma, angiomyofibroblastoma, fibroepithelial stromal polyp, intramuscular myxoma and myxoid variant of liposarcomas and fibrosarcomas [4]. The various differentiating histopathological features have been highlighted in Table 1.
Table 1: Histopathological& IHC characteristics of differential diagnosis of AA.
| Features |
Cellular AF |
Myxoid Neurothekoma |
Neurofibroma |
Superficial myofibroblastoma |
Angiomyofibroblastoma |
FE stromal polyp |
Intramuscular Myxoma |
Myxoid variant of Liposarcoma |
Myxoid variant of Fibrosarcoma |
AgressiveAngiomyxoma |
| Age |
4th decade |
< 3rd decade |
1st decade |
5th - 8th decade |
4th-5th Decade |
1 month, adult women |
5th - 6th decade |
4th - 5th decade |
<5 years |
young, middle aged |
| Gender |
> women |
> women |
No gender prediliction |
> women |
> women |
neonates, pregnant women |
women |
No gender prediliction |
No gender prediliction |
women |
| Site |
vulva, scrotum, inguinal region |
Head & upper extremities |
Any site |
cervico-vaginal region |
Vulvo-vaginal area |
Intertriginous areas, face, neck,eyelids |
Thigh, shoulder, pelvis |
Extremities, proximal thigh |
Extremities |
pelvic & perineal region |
| Circumscription |
well circumscribed |
multinodular |
unencapsulated |
well circumscribed |
well circumscribed |
well circumscribed |
well circumscribed |
poorly circumscribed |
poorly circumscribed |
poorly circumscribed |
| Cellularity |
moderately cellular |
cellular |
hypocellular |
moderately cellular |
hypo &hypercellualr areas |
hypocellular |
cellular |
paucicellular |
hypercellular |
hypocellular |
| Vessels |
Prominent vessels with hyalinized walls |
Inconspicuous |
Absent hyalinized vessels |
Absent hyalinized vessels |
Numerous vessels, few hayalinized |
Irregular shaped thin walled vessels |
inconspicuous vessels |
Branching (chicken wire) vasculature |
|
prominent thick walled |
| Mitotic figures |
Brisk mitosis in females |
10 + MF/25 HPF |
Rare |
Minimal |
Rarely |
Rare |
Rare |
Unusual |
Increased |
Rare |
| Atypical mitotic figures |
Absent |
may be + |
Absent |
Absent |
Absent |
Absent |
Absent |
Rare |
Present |
Absent |
| Infiltration in surrounding tissues |
Fat is integral to the lesio |
Infiltrative margins |
Infiltrative margins |
Absent |
Absent |
Absent |
Absent |
Present |
Present |
Infiltrative margins |
| Necrosis |
Absent |
Absent |
Absent |
Absent |
Absent |
Absent |
Absent |
Absent |
Absent |
Absent |
| Immunohistochemistry |
ER, PR, Vimentin |
Vimentin |
S- |
Vimentin, ER, PR |
Vimentin |
Vimentin+ |
Vimentin |
S-100 |
Vimentin, |
CD |
Imaging is usually not indicated in these cases, as majority present as benign polyps. It has a major role in deep seated lesions to visualise infiltration into surrounding soft tissue, involvement of underlying bone and encasement of any major vessel.On computed tomography (CT) scan, these tumors have a well-defined margin with attenuation less than or equal to that of the adjacent skeletal muscle with a swirled enhancing tissue internally [5]. On MRI scan, they show high signal intensity on T2-weighted images and a similar swirling. The attenuation on CT and high signal intensity on MRI are likely attributed to the loose myxoid matrix and high water content of angiomyxomas. Radiology was not conducted in the present case as the lesion presented as a pedunculated polyp.
Wide surgical excision with clear margins is the therapy of choice. The problem occurs as there are documented incidences of recurrences, especially following suboptimal resection. Practically, the complete excision of the tumour is difficult as the consistency of AA is almost the same as that of the normal connective tissue and in addition to that they are uncapsulated. The incidence of local recurrence is 36 to 72% and local recurrences cannot be predicted by either tumor size or cellularity. Chemotherapy and radiation therapy have not much role as the tumor has low mitotic potential. Treatment modalities like angiographic embolization of the mass; hormonal therapy with Tamoxifen, Raloxifen, Gonadotropin-releasing hormone agonist (GnRH-A) may be required in those circumstances in which the tumor has a limited success. In some reported cases pre-operative GnRH therapy has been tried in order to make complete resection easier and also to prevent recurrence [6]. GnRH therapy has also been used to treat tumor recurrence. Local excision of the tumour in the first setting forgetting the cosmetic value is ideal to prevent further recurrence, as has been done in our case too. There are no definite guidelines for post operative management and follow up of the vulvar AA but due to high incidence of recurrence, which may occur 2 months to 15 years after the initial resection, periodic check up is advocated both clinically and radiologically. MRI is the investigation of choice for the same. The patients are to be counselled properly and motivated for long term follow up. We are following up our patient for the past 10 months and fortunately she did not have any recurrence.
Conclusion
Aggressive angiomyxomas should be considered as an important differential in cases of slow growing vulvar masses even in children. Tumor has many morphological mimics which can be differentiated based on histomorphology and immunohistochemistry. Recurrence rates are very high and in view of this, long term surveillance is imperative and patient may be motivated to do the same.
Learning Points
1.AA is an important differential in cases of slow growing vulvar masses in children.
2.Detection of inappropriate HMGI-C expression using the immunoperoxidase technique is of proven value in histological differential diagnosis and also may potentially be a useful marker for microscopic residual disease.
3.AA has a wide histopathological differential diagnosis.
4.Local recurrence is high and cannot be predicted alone by cellularity or tumor size.
5.GnRH therapy preoperatively can be used to reduce the size, making resection easier and to treat tumor recurrence.
Guarantor of submission:
The corresponding author is the guarantor of submission
Conflict of Interest
There is no conflict of interest amongst the Authors
Author Contribution:
Manuscript drafting and assimilation: Dr. Nidhi Mahajan
Clinical inputs and images: Dr. C. R. GuptaData Collection: Dr. Mitali Agarwal
References
[1].Kawamura M, Matsumoto F, Matsui F, Yazawa K, Shimada K. Aggressive Angiomyxoma of the Vulva Mimicking Clitoromegaly in a Young Child. Urology. 2017 Mar;101:142-144.[PubMed]
[2].Das BP, Baruah D, Medhi KB, Talukder B. An aggressive angiomyxoma of vulva – A rare entity - A case report. J Midlife Health. 2016 Jul-Sep;7(3):140-143.{PubMed][PMC Full Text]
[3].T. A. Steeper and J. Rosai, “Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm,” American Journal of Surgical Pathology, vol. 7, no. 5, 463–475, 1983.[PubMed]
[4].Alameda F, Munne A, Baro T, Iglesias M, Condom E, Lloreta-Trull J, Serrano S Vulvar angiomyxoma, aggressive angiomyxoma and angiomyofibroblastoma:An immunohistochemical and ultrastructural study. Ultrastruct Pathol. 2006;30:193–205 .[PubMed] [Free Full Text]
[5].S. Srinivasan, V. Krishnan, S. Z. Ali, and N. Chidambaranathan, “‘Swirl sign’ of aggressive angiomyxoma—a lesser known diagnostic sign,” Clinical Imaging, vol. 38, no. 5, pp. 751–754, 2014.[PubMed] [Free Full Text]
[6].Srivastava P, Ahluwalia C, Zaheer S, Mandal AK. Aggressive angiomyxoma of vulva in a 13-year-old female. J Can Res Ther 2015; 11: 937-9.[PubMed][Free Full Text

