Case Report

table.MsoTableGrid
{border:solid windowtext 1.0pt;
font-size:11.0pt;
font-family:"Calibri","sans-serif";
}
Primary Leiomyosarcoma of the Breast with Axillary Nodal Metastasis : A Case Report and Review of Literature
1 Shah Naveed 2 Ghanish Panjwani 3 Hasina Qari 4 BB Panday
- 1Deptt. Of Surgical Oncology Mahavir Cancer Sansthan INDIA.
- 2Deptt. Of Surgical Oncology Mahavir Cancer Sansthan INDIA
- 3Deptt. Anesthesia IGIMS INDIA.
- 4ProffesorDeptt. Of Surgical Oncology Mahavir Cancer Sansthan INDIA
- Submitted: Thursday, April 14, 2016
- Accepted: Friday, August 19, 2016
- Published:Sunday, August 21, 2016
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ((http://creativecommons.org/licenses/by/3.0)which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Introduction
Primary breast sarcomas are rare tumors. They originate from the mesenchymal tissue of the breast. The prevalence of primary breast sarcomas as reported at Mayo Clinic among breast cancers was found to be 0.0006% [1]
Among sarcomas of breast primary leiomyosarcoma iseven more rarely seen and less than 50 cases have been reported in the world literature. Most of the reported cases are between the age of fifty and eighty yearsand occurs usually in postmenopausal women. Most cases are low-grade and are cured by complete excision with wide margins.In the present study, we present a case of primary leiomyosarcoma of the breast which had axillary metastasis, and the first such case reported in literatureand we discuss optimal treatment options.
Case Presentation
A 40-year-old female patient was referred to our hospital because of a big ulcerated mass in the breast (Figure 1). She noticed a lump in her right breast 1 year ago and was on some ayurvedic medicine, but recently this lump had grown rapidly and led to the ulceration of the skin . Physical examination revealed 25/25 cm ulcerated lesion in thebreast. There was no retraction of the nipple but had palpable axillary nodes 2×2 cm. The contralateral breast was normal. Mammography detected a large mass (BIRADS 5).
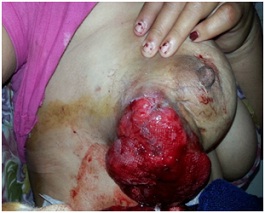
Figure 1: Leimyosarcoma Breast
Core needle biopsy of the right breast revealed a spindle-cell neoplasm composed of tumor cells with blunt ended nuclei that were strongly positive for smooth muscle actin (SMA) and vimentin, and lacked expression of pan-cytokeratin, CD34, and S-100. This immunophenotype is most consistent with a diagnosis of breast sarcoma.
Microscopically, the tumor was composed of pleomorphic spindle cells showing frequent mitoses and necrosis. Tumor cells demonstrated diffuse immunohistochemical staining with smooth muscle actin (SMA). No staining was observed with desmin, S100, CD117. According to the histopathological and immunohistochemical analysis, the tumor was diagnosed as leiomyosarcoma.FNAC of the axillary nodes showed metastatic sarcoma. Modified radicalmastectomy was done as the tumor was large and had axillary metastasis.The patient is doing well and on regular follow up.
A follow-up thoracoabdominal computed tomography scan and bone scintigraphy performed 1 month after the operation were normal
Discussion
Primary leiomyosarcoma of the breast is an extremely rare malignant neoplasm of uncertain biological behavior. There are less than 50 well-documented cases reported in the English medical literature [1]. As this tumor is rare more patient data should be presented for the development of a proper treatment strategy.
The majority of these cases present as a well-circumscribed mass in the breast of postmenopausal women. The exact origin of the entity is not clear. The myofibroblasts in the nipple areola complex have been proposed as the origin for the neoplasm [2]. The mainstay treatment is wide margin local excision. Most reported cases have undergone mastectomy with a few exceptions being treated with lumpectomy. Axillary dissection is believed to be unnecessary as the primary leiomyosaroma of the breast does not spread through the lymphatic route, but as in our case it had metastasized to axillary nodes MRM was necessary and ours is first such case reported in literature.
To date, this type of tumor has been observed to affect middle-aged women and manifests as a mass in their breast over a long period of time. The average tumor size is 4.7 cm [3, 4]. The involvement of endocrine factors in this tumor appears to be unlikely as the two reported cases were in males [3]. This tumor tends to show local recurrence and distant metastasis has been observed in 25% of patients [5, 6]. Metastatic spreading usually occurs via the hematogenous route. Tumor size is believed to have no significant correlation with metastasis [5]. The studies published to date show that the tumor presents itself as a long-standing and slow-growing mass. The duration of symptoms varies between 2 weeks and 5 years, confirming those characteristics [5]. Local recurrence and distant metastases can arise even after 15-20 years [7]. Chen et al. [8] and Nielsen [9] lost their patients 16 and 20 years, respectively, after the operation due to hepatic and multiple metastases.
Leiomyosarcoma is characterized by spindle-shaped cells with pleomorphic, hyperchromatic and elongated nuclei; eosinophilic cytoplasm; large nucleoli; and significant mitoses. Definitive diagnosis is established through histological examination, in which positive staining is observed immunohistochemically with desmin, vimentin, and muscle-specific actin, whereas negative staining is seen with cytokeratin, myoglobin, and S-100 [10]. Leiomyosarcoma are generally strongly and uniformly positive for SMA and HHF35 (Figure 2).
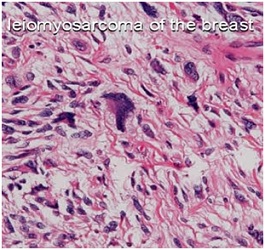
Figure 2: Photomicrograph showing tumor composed of spindle cell: leiomyosarcoma
Smooth muscle actin is more specific for smooth muscle than panactin HHF35. It is usually negative in skeletal muscle tumors in contrast to HHF35, which is frequently positive in these tumors. Desmin positivity varies with leiomyoma being usually at least focally positive, whereas LMS is positive in only 70% to 80% of cases, with less staining in the poorly differentiated examples.Calponin, a cytoskeleton-associated actin-binding protein, is frequently used as a myoepithelial marker but is also very useful in detecting smooth muscle differentiation in STTs. In addition to smooth muscle and myoepithelial cells, it is also expressed in myofibroblasts. It is consistently positive in leiomyoma, and it is positive in a high percentage of LMSs (90% in conventional LMS, 70% in pleomorphic LMS). To define a poorly differentiated spindle cell sarcoma as LMS, at least 2 of 3 muscle markers (using for example SMA/desmin/HHF35 or SMA/desmin/calponin) should be positive, and the H&E appearance should be supportive as well. Markers specific for skeletal muscle differentiation (myoglobin, myogenin) are consistently negative in LMS. Keratins and EMA are positive in 10% to 30% of LMSs [11]. Because leiomyosarcoma often invades peripheral tissues, such as the skin and fascia, curative surgery requires a wide resection. Because there has been no reported cases of lymph node metastasis in which dissemination occurred via the hematogenous route, axillary lymph node dissection is unnecessary if leiomyosarcoma diagnosis can be achieved before the operation [10], but our case is the first to show axillary nodal metastasis and thus importance of preoperative FNAC for any palpable axillary lymphadenopathy. Prognosis is determined primarily by the adequacy of surgical resection. Although, there is no definite consensus on the use of adjuvant chemotherapy or radiotherapy, most patients reported till date have done well without any chemotherapy or radiotherapy, at least in the initial few years (Table 1) . In the present case, there was axillary lymph node involvement. MRM was performed due to large ulcerated mass with invasion of the pectoralis major muscle by the tumor. The prognosis of leiomyosarcoma is better than that of other breast sarcomas [5]. The prognostic factors are not fully known because of the limited number of studies
|
Author
|
Year
|
Age/Sex
|
size (cm)
|
Mitosis (/10hpf)
|
Treatment
|
Ct/Rt
|
Final follow up
|
|
Haagensen
|
1971
|
77/F
|
8
|
Very frequent
|
SM
|
-
|
Alive, 14 yrs
|
|
PardoMindan et al.
|
1974
|
49/F
|
7
|
16
|
SM
|
-
|
Alive, 6 months
|
|
Barnes and Pietruszka
|
1977
|
55/F
|
3
|
10
|
SM
|
CT
|
Died 4 yrs later
|
|
Hernandez
|
1978
|
53/M
|
4
|
15
|
MRM
|
-
|
Alive 1 yr
|
|
Chen et al.
|
1981
|
59/F
|
5.6
|
3
|
SM
|
CT
|
Alive 15yrs
|
|
Callery et al.
|
1984
|
56/F
|
2
|
-
|
SM
|
-
|
Alive 39 months
|
|
Callery et al.
|
1984
|
54/F
|
3
|
-
|
SM
|
-
|
Alive 53 months
|
|
Yatsuka et al.
|
1984
|
56/F
|
1.5
|
21
|
RM
|
-
|
Alive 4 yrs
|
|
Gobardhan
|
1984
|
50/F
|
9
|
5
|
MRM
|
-
|
Alive 2 yrs
|
|
Nielsen
|
1984
|
24/F
|
1.5
|
2
|
WLE
|
-
|
Died 20yrs later
|
|
Yamashina
|
1987
|
62/F
|
2.5
|
11
|
SM
|
-
|
Alive 2yrs
|
|
Arista-Nasr et
|
1989
|
50/F
|
4.5/2.3
|
4
|
WLE
|
-
|
Alive 6yrs
|
|
Parham et al.
|
1992
|
52/F
|
3
|
29
|
SM
|
RT
|
Alive 6 months
|
|
Lonsdale and Widdison
|
1992
|
60/F
|
2
|
10
|
SM
|
-
|
Alive 3 months
|
|
Waterworth et al
|
1992
|
58/F
|
4
|
10
|
WLE+AC
|
-
|
Alive 1yr
|
|
Wei et al.
|
1993
|
36/F
|
4
|
-
|
MRM
|
-
|
Died 14 months later
|
|
Boscaino et al.
|
1994
|
56/F
|
2.5/4
|
2
|
WLE
|
-
|
Alive 9 yrs
|
|
Boscaino et al.
|
1994
|
45/F
|
1.9
|
2
|
WLE
|
-
|
Alive 40 months
|
|
Levy et al.
|
1995
|
35/F
|
4
|
2
|
SM
|
-
|
Alive 6 months
|
|
Falconieri et al.
|
1997
|
83/F
|
6
|
20
|
RM
|
RT
|
Alive 10 months
|
|
Falconieri et al.
|
1997
|
86/F
|
8
|
11
|
SM
|
-
|
Alive 8 months
|
|
Ugras et al.
|
1997
|
47/F
|
2
|
3
|
SM
|
-
|
Alive 1.5yrs
|
|
González-Palacios
|
1998
|
62/F
|
3
|
10
|
SM
|
Paliative CT
|
Alive 17yrs
|
|
Gupta et al.
|
2000
|
80/F
|
6.5
|
5-8
|
SM+AC
|
-
|
Alive 2yrs
|
|
Székely et al.
|
2001
|
73/F
|
4.8
|
20-22
|
SM
|
-
|
Alive 1yr
|
|
Kusama et al.
|
2002
|
55/F
|
0.5
|
few
|
WLE
|
|
Alive 4yrs
|
|
Shinto et al.
|
2002
|
59/F
|
12
|
19
|
SM
|
|
Alive 8 months
|
|
Wei et al.
|
2003
|
52/F
|
4
|
22
|
WLE
|
|
Alive 3 months
|
|
Markaki et al.
|
2003
|
42/F
|
14
|
50
|
MRM
|
|
Alive 3yrs
|
|
Markaki et al.
|
2003
|
65/F
|
5.2
|
10
|
E
|
|
Alive 18 months
|
|
Liang et al.
|
2003
|
25/F
|
4
|
5
|
E
|
|
Alive 32 months
|
|
Adem et al.
|
2004
|
67/F
|
2
|
-
|
E
|
|
Died 7 months later
|
|
Adem et al.
|
2004
|
55/F
|
4
|
-
|
SM
|
|
Died 77 months later
|
|
Jayaram et al.
|
2004
|
55/F
|
12
|
-
|
MRM
|
|
Local recurrence
|
|
Lee et al.
|
2004
|
44/F
|
3
|
6-12
|
SM
|
|
Alive 13months
|
|
Lee et al.
|
2004
|
52/F
|
4.5
|
6-12
|
SM
|
|
Alive 17 months
|
|
Stafyla et al.
|
2004
|
53/F
|
23
|
-
|
MRM
|
|
Alive 2yrs
|
|
Munitiz et al.
|
2004
|
58/F
|
4
|
14
|
MRM
|
|
Alive 1yr
|
|
Gupta
|
2006
|
37/F
|
8
|
15
|
WLE
|
|
Alive 36 months
|
|
Vu et al.
|
2006
|
-/F
|
23
|
-
|
SM
|
|
Alive 10 months
|
|
De la Pena and Wapnir
|
2008
|
50/F
|
3.2
|
-
|
SM
|
|
Alive 11 months
|
|
Wong et al.
|
2008
|
52/F
|
1.5
|
7
|
SM
|
|
Alive 4 days
|
|
Cobanoglu et al.
|
2009
|
64/F
|
3.5
|
12
|
MRM
|
|
Alive 22 months
|
|
Fujita et al.
|
2010
|
18/F
|
7.2
|
10
|
SM
|
|
Alive 5 yrs
|
|
Swapnil et al
|
2011
|
19/F
|
7
|
20-25
|
WLE
|
|
Alive 3yrs
|
|
Zulfikar et al
|
2012
|
48/F
|
10
|
-
|
MRM
|
|
Alive 1 month
|
|
Amaadour et al
|
2013
|
44/F
|
9.2/7.6cm
|
|
-
|
|
Died after 1 month
|
|
Sokolovskayaet al
|
2014
|
58/F
|
15/15cm
|
-
|
MRM
|
|
Alive after 2 years
|
|
Present Case
|
2015
|
40/F
|
25/25cm
|
|
MRM
|
|
Alive after 1 year
|
They require long-term follow-up because local recurrence and distant metastasis can occur long after the operation. After surgical resection, late local recurrence and distant hematogenous metastasis to lungs and liver is, however, well-documented and bone metastasis has once been reported [12]. It has a better prognosis than other breast sarcomas. However, there is a need for further studies to determine the prognostic factors.
Conclusions
Leiomyosarcoma of the breast is a rare entity with patients typically being in the 5th−7th decade. Morphologically it can be suspected by the typical histological features of circumscription, high cellularity and being composed of fusiform spindle cells having blunt end nuclei. Confirmation by an immunohistochemical profile of smooth muscle actin, vimentin, and desmin positivity is helpful. The mainstay treatment is wide margin local excision. Axillary dissection is believed to be unnecessary as the primary leiomyosaroma of the breast does not spread through the lymphatic route.
Author’s Contribution
SN: Collection of data and involved in editing the article.
GP: Helped in finding the previous research material for the
article.
HQ: involved in collection of data and final editing of the
article.
BBP: Helped in analyzing the histopathological and
Immunohistochemistry.
Conflict of Interest
The authors declare that there are no conflict of interests.
Ethical Considerations
The written informed consent was obtained from the patient for publication of this case report. The copy of the consent is available with the authors.
References
[1]Nagao T, Hojo T, Tanaka-Akashi S, et al.: Primary leiomyosarcoma of the breast. Breast J. 2012; 8(1): 81–82 [PubMed]
[2]. Cameron HM, Hamperl H, Warambo W: Leiomyosarcoma of the breast originating from myothelium (myoepithelium). J Pathol. 1974; 114(2): 89–92 [PubMed]
[3]. Munitiz V, Rios A, Canovas J, Ferri B, Sola J, Canovas P, et al. Primitive leiomyosarcoma of the breast: case report and review of the literature. Breast. 2004; 13:72–76. [PubMed]
[4]Shinto O, Yashiro M, Yamada N, Matsuoka T, Ohira M, Ishikawa T, et al. Primary leiomyosarcoma of the breast: report of a case. Surg Today. 2002;32:716–719. [PubMed]
[5]Levy RD, Degiannis E, Obers V, Saadia R. Leiomyosarcoma of the breast.A case report. S Afr J Surg.1995; 33:15–17 [PubMed]
[6]Waterworth PD, Gompertz RH, Hennessy C, Henry JA, Lennard TW. Primary leiomyosarcoma of the breast. Br J Surg. 1992; 79:169–170 [PubMed]
[7]Hussien M, Sivananthan S, Anderson N, Shiels A, Tracey N, Odling-Smee GW. Primary leiomyosarcoma of the breast: diagnosis, management and outcome. A report of a new case and review of literature. Breast. 2001; 10:530–534 [PubMed]
[8]Chen KT, Kuo TT, Hoffmann KD. Leiomyosarcoma of the breast: a case of long survival and late hepatic metastasis. Cancer. 1981; 47:1883–1886 [PubMed]
[9]Nielsen BB. Leiomyosarcoma of the breast with late dissemination. Virchows Arch A PatholAnatHistopathol. 1984;403:241–245 [PubMed]
[10]ZülfikarKarabulut, HamparAkkaya, and Gökhan MorayPrimary Leiomyosarcoma of the Breast: A Case ReportJ Breast Cancer. Mar 2012; 15(1): 124–127.[PubMed]
[11]Josefine Heim-Hall, MD; Sophia L. Yohe, MD: Application of Immunohistochemistry to Soft Tissue Neoplasms Arch Pathol Lab Med—Vol 132, March 2008 [PubMed]
[12]Sokolovskaya E, Liu Z, Weintraub K et al. Case Report: Primary Leiomyosarcoma of the breast with unusual metastasis to the femur. F1000Research 2014, 3:211.[PubMed]

