Case Report

Anteriorly Based Tongue Flap for Closure of a Posterior Palatal Defect in a Patient with Exaggerated Gag Reflex
1 Robert A. Beech,2 John Reid 3 Leslie Heffez,4 Henry Fung
- 1Senior Resident,
- 2Chief Resident 2012-2013, Oral &Maxillofacial Surgery Department, Cook County Hospital, 1900 W. Polk St. Ste 611, Chicago, IL 60612, USA,
- 3Former Professor and Head University of Illinois at Chicago, Department of Oral and Maxillofacial Surgery. Currently in Private Practice. Volunteer Faculty Cook County Stroger Hospital Oral and Maxillofacial Surgery. Chicago, I
- 4Division Chair &Program Director of Cook County Stroger Hospital Oral and Maxillofacial Surgery
- Submitted: Thursday, May 21, 2015
- Accepted: Friday, August 07, 2015
- Published: Friday, September 25, 2015
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ((http://creativecommons.org/licenses/by/3.0)which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Background
Closure of palatal fistulas requires meticulous attention to detail as well as sound understanding of relevant anatomy. Tongue flaps of various design based on defect location, size and confounding patient factors, have been proven to be effective in closing intraoral defects.
Case Description
A 37 year old Hispanic female presented to our hospital with a palatal pleomorphic adenoma. After initial resection, a split thickness skin graft was performed to the operative site, and prosthesis was fabricated. The patient could not tolerate her obturator secondary to a severe gag reflex, and was left with residual 3x2cm oro-nasal defect. Reconstruction of the defect was planned with the use of an anteriorly based tongue flap due to concerns that the patient’s severe gag reflex would make a traditional posteriorly based flap intolerable.
Practical Implications
The purpose of this article is to describe the options available for closure of an oro-nasal fistula, and describe our use of an anteriorly based dorsal tongue flap to close a large posterior soft palate defect in a patient with a severe gag reflex. The exaggerated reflexes might have otherwise been considered a contraindication.
Introduction
Tongue flaps have been used in the field of oral and maxillofacial reconstruction over the past 100 years. Since the first reports in the literature, there have been multiple modifications in techniques and flap designs that documented favorable results [1 2]. In 1909, Lexer [3] documented the first use of a posteriorly based dorsal tongue flap for the management of retro-molar and tonsillar defects. In 1956, Klopp and Schurter [4] reconstructed a soft palate defect secondary to excision of malignancy with a posteriorly based lateral tongue flap. Guerrero-Santos
et al., [5] published a series of case reports describing the use of tongue flaps in lip reconstruction in 1964. Two years later, these authors documented success with the use of a dorsally based tongue flap for closure of palatal defects caused by bullet wounds, tumors, and clefts.
Vascular studies by Bracka [6] have demonstrated the rich vascularity of the tongue and has shown the posteriorly based tongue flap to have the most reliable blood supply based off the dorsal lingual artery. The design of the posteriorly based tongue flap is, however, slightly limited due to its lack of mobility and close approximation to the circumvallate papilla. When elevating this flap, careful attention must be paid to not extend past the circumvallate papilla, which could result in compromise of the blood supply. The laterally based tongue flap and anteriorly based dorsal tongue flap are two additional flap designs that have been extensively reported in the literature and have been used to successfully close a variety of intraoral defects of various etiologies. The anteriorly based dorsal tongue flap is considered to be the most versatile of the tongue flaps due to its superior mobility and its rich sub-mucous vascular plexus as described by Cadenat
et al [7].
The following is a report of the author’s experience using an anteriorly based dorsal tongue flap to treat a posterior palatal defect in a patient with an exaggerated gag reflex.
Case Report
A 37 year old otherwise healthy Hispanic female presented to the outpatient clinic at Cook County Hospital in Chicago Illinois with a chief complaint of a slow growing left-sided intraoral palatal mass of approximately 5 years duration. Initial evaluation revealed an intraoral fixed, non-ulcerated and non-tender soft tissue mass at the junction of the hard and soft palate measuring 2.8 X 2.5cm. Head and
neck exam was unremarkable with no signs of lymphadenopathy and a normal maximal incisal opening of 45mm. The patient was subsequently set up for a CT and MRI which both showed a well demarcated soft tissue mass of the soft palate with no evidence of bony invasion or nodal pathology (Figure1,2,3) Both studies were evident of a benign soft tissue process. Incisional biopsy revealed pleomorphic adenoma. A decision was made following baseline speech therapy evaluation for resection of the lesion and placement of a split thickness skin graft at the margins to prevent scar contracture and prevent any negative consequences to her speech. Once the surgical procedure was performed an interim prosthesis was fabricated by a prosthodontist.
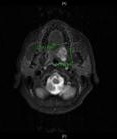
Figure1: Axial MRI T2 weighted image showing 2.3 cm X 2.4 cm lesion of the soft palate.
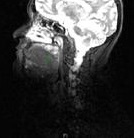
Figure2: Sagittal MRI T1 weighted image showing well demarcated lesion of hard/soft palate.
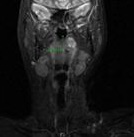
Figure3: Coronal MRI T2 weighted image: 1.8cm vertical height
On follow up the patient was unable to tolerate her obturator prosthesis. After 7 months of healing, the patient’s residual oro-nasal defect clinically measured 3.0 cm X 2.0 cm (Figure. 4). Reconstruction of the defect was planned with the use of an anteriorly based tongue flap due to concerns that the patient’s severe gag reflex would make a posteriorly based flap intolerable.
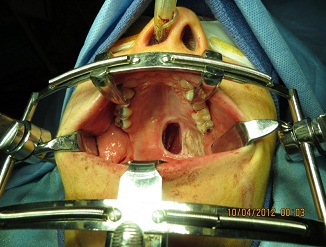
Figure 4: 3 X 2 cm defect of left soft palate. Dense scar tissue formation at left margin from previous skin grafts at time of ablative surgery.
The reconstruction was performed under general anesthesia. A Dingman cleft palate mouth gag was used to expose the soft palate defect and retract the tongue inferiorly. The recipient site was prepared by performing oral mucosal incisions 7 mm around the periphery of the defect for the purpose of inverting the oral edges into the nasopharynx (Figure. 5).

Figure 5: Mucosal incision was performed surrounding the periphery of the defect. Submucosal undermining was performed to develop the tissue bed for the graft.
The Dingman retractor was then removed. An anteriorly based tongue flap was then elevated within the midline of the dorsum of the tongue 1.0 cm anterior to the circumvallate papilla. Approximation of the undermined mucosal tissue at the defect site was completed in a tension-free manner to act as a nasal epithelial layer to adhere to the transported tongue flap (Figure. 6).
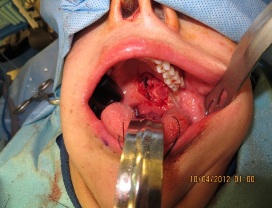
Figure 6: Undermined margins sutured together to develop the tissue bed and act as a nasal mucosal layer for double layered closure.
The flap thickness was maintained at approximately 10 mm (Figure. 7). The flap was lifted into place with epithelium facing the oral cavity. The anterior and lateral aspects of the defect were sutured using interrupted vertical mattress sutures with 3-0 vicryl (Figure. 8).
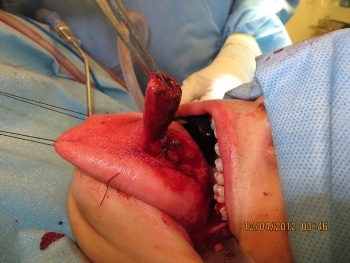
Figure 7: Anteriorly based tongue flap raised showing muscle thickness utilized
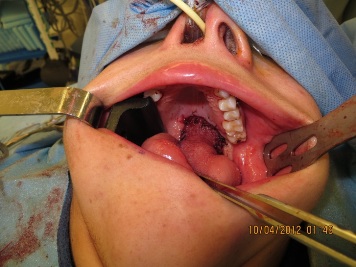
Figure 8: Flap closure at time of surgery. Posterior root of the flap is not sutured and risks residual fistula to be closed at the time of division of the pedicle.
The donor site was closed primarily only in its most posterior aspect. The patient was then placed into maxilla-mandibular fixation with ivy loops to reduce the effect of tongue stimulation on the gag reflex. Following an uneventful extubation, she was transitioned to light elastics to limit her maximum incisal opening. She received Dobhoff tube feedings for a total of 3 weeks to both limit initial aggravation of the gag reflex during swallowing, and to prevent contamination of the flap. Subsequently, she was able to tolerate a full liquid diet for 2 weeks. At week 3 post operatively, the viability of the flap was confirmed using an iodoform gauze tourniquet. Delayed surgical release of the flap was performed under general anesthesia at week 5 due to scheduling issues (Figure.9, 10).
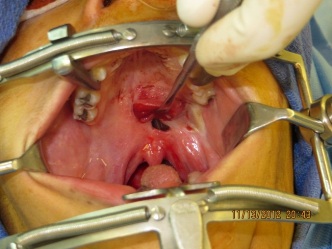
Figure 9: 5mm defect remaining at posterior ledge following release of the flap at 5 weeks. Note the good vascularity of the flap.
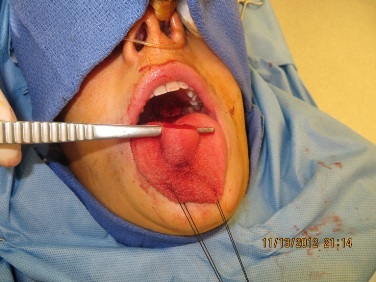
Figure 10: Flap base following release.
At the time of release, a sevoflurane breath-down technique was used and the flap was released with a # 15 blade. Nasal intubation was performed immediately following this release via direct laryngoscopy. As expected, there was a 5mm x 5mm residual defect at the root of the pedicle where it was rotated onto the palate. The defect margin in this area was de-epithelialized and primary closure was obtained (Figure 11,12). She was followed on a weekly basis and showed no signs of tissue breakdown or recurrent fistula at her 7 months post-operative appointment (Figure.13). The patient was referred for evaluation with a Speech Pathologist, and her speech was deemed unaltered following her reconstruction.
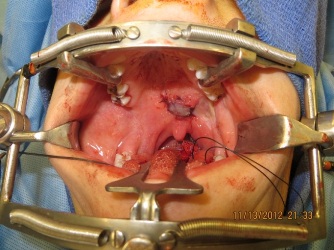
Figure 11: Closure of the remaining posterior defect.
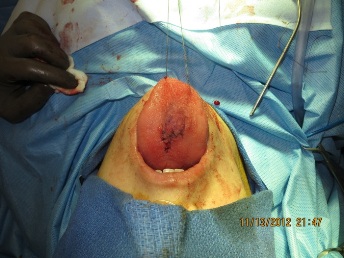
Figure 12: Flap base inset into donor site.
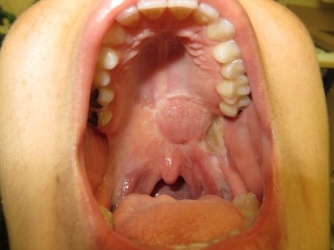
Figure 13: 7 months post op. Good integration of the flap with no signs of residual fistula formation. Uvula is midline and the flap is well vascularized.
Discussion
Reconstruction of small to moderate sized palatal defects can be achieved by a variety of local flaps including: the palatal island flap [8], buccinator myomucosal flap [9], buccal fat pad flap [10], and a variety of tongue flap designs including anterior, posterior and laterally based flaps (Figure 14) (Figure 15) [11 12]. With intraoral defects larger than 1.5 cm in diameter, the use of local buccal or palatal flaps is technically difficult, and closure may be inadequate due to the limited quantity of suitable donor tissue. It has been the author’s experience that larger defects measuring 1.5 cm to 3.5 cm in diameter are best reconstructed with dorsal tongue flaps due to the abundance of tissue and the rich vascular supply.
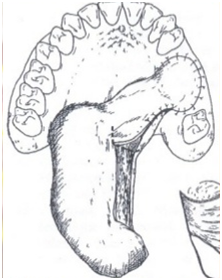
Figure 14: A laterally based posterior tongue flap being utilized for left maxillary oral-antral communication closure
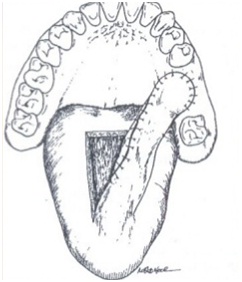
Figure 15: A dorsal anteriorly based tongue flap being used for left maxillary oral-antral communication
Indications for the use of tongue flaps are diverse and include defects of the tonsillar area, tongue base, soft palate, buccal mucosa and floor of the mouth. Contraindications include large pharyngeal resection defects that extend into the piriform sinus, smaller defects that could be closed with simple skin grafts, or surface defects that can allow to be closed by secondary intention.
When planning the design of a dorsal tongue flap for the closure of oral-nasal or oral-antral fistulas, the surgeon should consider the location of the defect. General parameters utilized in flap design include: diameter 20% greater than the defect’s to obtain a tension-free closure and thickness minimum of 3 mm to avoid damaging the submucous vascular plexus. When more bulk is needed, flaps of 10 mm thickness have been designed to include up to 2/3 of the dorsum of the tongue [13]. Posteriorly based flaps are usually best suited for defects of the soft palate, retro-molar region or posterior buccal mucosa. For defects located on the hard palate, anterior buccal mucosa, anterior floor of the mouth and lips, anteriorly based flaps are typically recommended [14].
Following the surgical procedure, the authors have found that use of light inter-maxillary fixation can aid in the promotion of revascularization and wound healing, due to decreased mandibular movement and a reduction in shearing forces. Initial dobhoff tube feeding is beneficial; aiding in both reduction of graft mobility during mastication, as well as prevention of surgical site contamination. Feeding typically begin 5-7 days post operatively if there is no sign of infection or fistula. Many times if the tongue flap is in coordination with a primary cancer resection, a tracheostomy is required for protection of the airway. The tongue flap may commonly look dusky or unhealthy following surgery, however if no fistula formation occurs in the early post-operative course the flap is deemed successful [15].
Following fistula closure our patient was evaluated at 1 week, 3 weeks, 5 weeks, 2 months, 4 months, and 7 months post operatively. In this surgical patient, flap viability was confirmed at 3 weeks. If however, the tongue flap demonstrates congestion, systemic heparin can be administered until venous outflow can be re-established. Alternatively, pin pricking the flap can be beneficial to venous outflow. The adjunctive treatment with hyperbaric oxygen for flaps lacking sufficient blood flow can also be considered. Any obvious necrotic areas can be trimmed away under local anesthetic as needed. Once viability in our patient was confirmed flap division then took place at 5 weeks post-operatively.
The patient was followed post-operatively by a speech pathologist. Speech and swallowing problems are typically related more to tongue mobility than the size of the tongue flap. Swallowing is rarely altered, however speech impairment can arise when the tongue flap is rotated into the check or floor of the mouth [15].. The difficulty with phonation is more obvious early in the post-operative course, commonly improving over time. Our patient’s speech intelligibility, nasal emissions, and hyper-nasality were assessed. She was encouraged to perform palatal muscular strengthening exercises involving blowing up balloons, sucking through straws, and direct finger stimulation of the area. Speech assessment confirmed that the patient’s speech had returned to baseline, with the added benefit of no longer being dependent on a poorly tolerated prosthesis.
Summary
Tongue flaps have a long standing history in oral and maxillofacial reconstruction and have been found to be extremely successful with minimal morbidity. These flaps should be selected based on defect characteristics and patient factors. The authors feel that this case demonstrates the reliability and versatility of the anteriorly based dorsal tongue flap which can be utilized in patients with an exaggerated gag reflex. While anteriorly based flaps are usually indicated to reconstruct defects of the anterior intraoral region, this case illustrates a situation where such design can be used to close a large posterior-lateral soft palate defect. As chronic postoperative gagging with the less mobile posteriorly based flap would have risked pulling at the suture margins, this design allowed for both patient comfort and wound healing.
A patient with a severe gag reflex complicates the available options for reconstruction and results in greater surgical morbidity rates. To accommodate the patient’s sensitivity an anteriorly based tongue flap proved to be a viable option for such posterior oropharyngeal defects. These flaps, when appropriately planned, can significantly improve the quality of life in many patients.
Author Contribution
RB: Primary Author, In charge of article submission, Junior resident of Oral Surgery at time of patients hospital care, Acquisition of Data.
JR: Secondary author. Chief resident of Oral Surgery during patient’s care at our hospital. Data Acquisition.
LH: Editor. Primary Oral Surgery attending of record at time of patients care.
HF: Editor. Chairman of Oral Surgery Program at time of patients care.
Conflict of interest
All authors involved do not have any declarations nor conflicts of interest to document.
Patient Consent
Neither patient names, nor faces were used in the presentation of this case report. The patient involved is aware that her case is being presented for publication, and freely gave consent to publish the details of her surgery.
Funding
None declared
References
[1]Conley JJ, De Amesti F, Pierce MK. The use of tongue flaps in head and neck surgery. Surgery 1957; 41:745-8 [PubMed]
[2]Bakamjian VY. Use of tongue flaps in lower-lip reconstruction. Br. J of Plastic Surg 1964; 64 17:76-9 [PubMed]
[3]Lexer E: Wan genplastik. Dtsch. Z. Chir. (Leizpg) 1909; 100:206
[4].Klopp CT, Schurter ER: The surgical treatment of cancer of the soft palate and tonsil. Cancer 1956; 9: 1239. [PubMed]
[5]Guerrero-Santos J, Vasquez-Palliares R, Vera A, Machain P, Castaneda A: Tongue flap reconstruction of the lip. Trans 3rd Int Cong Plast Surg. P 1055. Excerpta Medica Found Amsterdam, 1964
[6]Bracka A. The blood supply of dorsal tongue flaps. Br J Plastic Surg 1981; 34:379-84 [PubMed]
[7]Cadenat H, Combelle R, Fabie M. Lambeaux de langue, vascularisation, morphologic et utilization. Ann Chir Plast 1973; 18:223-7 [PubMed]
[8]Moore BA, Magdy E, Netterville JL, Burkley BB. Palatal reconstruction with the palatal island flap. Laryngoscope. 2003 Jun; 113 (6):946-51 [pubMed]
[9]Bozola AR, Gasques JA, Carriquiry CE, et al: The buccinator musculomucosal flap: Anatomic study and clinical application. Plast Reconstr Surg 84:250, 1989 [PubMed]
[10]Abbas K, Behnam B, Mohammad M, Mohammed F, Nima M. Effectiveness of buccal fat and closing residual mid palatal and posterior palatal fistulas in patient’s previously treated for clefts. Journal of Oral and Maxillofacial Surgery 2011; 69:e416-419. [PubMed]
[11]Posnick JC, Getz SB. Surgical closure of end-stage palatal fistulas using anteriorly based dorsal tongue flaps. J Oral Maxillofac Surg 1987; 45:907-12 [PubMed]
[12]Buchbinder D, St-Hilaire H. Tongue Flaps in Maxillofacial Surgery. Oral and Maxillofacial Surgery Clinics of North America 2003 Nov:15(4):475-86 [PubMed]
[13]Smith TS, Siegfried J, Collins John T. Repair of a palatal defect using a dorsal pedicle tongue flap. J Oral Maxillofac Surg 1982; 40:670-673 [PubMed]
[14]Sathish M.S. Vasishta et al. The Versatility of the Tongue Flap in the Closure of Palatal Fistula. Craniomaxillofac Trauma Reconstr. 2012 Sep; 5(3): 145–160.[PubMed]
[15].Bailey, Byron. Atlas of Head & Neck Surgery—Otolaryngology. Section 1 Head and Neck. Oral Cavity. Page 104. 2001 [PubMed]

