Case Report

Insular Thyroid Microcarcinoma with Precedent Adenopathy – Peculiar Subset of Malignant Tumor
1Mihai Radu Diaconescu, 1Mihai Glod, 1Mirela Grigorovici, 2Smaranda Diaconescu
- 1IVst Surgical Clinic, "Gr T Popa" University of Medicine and Pharmacy Iasi, 700507, Romania
- 2Vth Pediatric Clinic, “St Maria” Children’s Emergency Hospital; 62, Vasile Lupu str, Iasi 700309
- Submitted: February 17 2013;
- Accepted: March 29 2013;
- Published: April 01 2013;
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
A case report of a patient with insular thyroid carcinoma, a poorly differentiated subtype of thyroid tumor with intermediate aggressiveness and prognosis between well-differentiated and anaplastic thyroid malignant lesions is presented.
Case Report
A 59 year old man with an apparent isolated huge lateral cervical adenopathy was directed to our clinic. The thyroid and other cervical structures were clinically inapparent and also could not be identified by imaging methods. Fine-needle aspiration biopsy showed aggregates of small, monomorphic cells with scant cytoplasm, round nuclei with granular chromatin and rare mitosis oriented the diagnosis towards a follicular thyroid carcinoma metastasis.
While intraoperatively completing the selective cervical dissection, a solitary centrolobar micronodule (5 mm) was identified and a total thyroidectomy was done. Microscopy showed neoplastic cells arranged in nests (insulae) separated by hyaline stroma, with grooved nuclei, rare mitosis and necrotic areas diagnostic for insular carcinoma. Complementary radioiodine therapy allowed a stable cure at 36 months follow-up.
Discussion
Appearance of these tumors with rapid evolution in which only precocious diagnosis (although not always possible) orienting the patient towards radical surgery completed by 131 I therapy, both assuring in 1/3 of cases a real chance of cure, to the poorly differentiated thyroid carcinomas is underlined.
Conclusion
Together with the rarity of this subtype of thyroid neoplasia, must be mentioned the precedency of the cervical adenopathy to the primary subclinical microcarcinoma and also the efficacy of the association between radical surgery with 131 I radiation.
Key Words
Insular thyroid microcarcinoma, fine needle-biopsy, prognosis, total thyroidectomy.
Background
Thyroid carcinomas include a lot of tumors with raised incidence/prevalence (the most frequent malignant lesion of the endocrine system) with a broad diversity of the clinical and evolutionary aspects of the biologic behavior deriving from their extreme histological polymorphism. Natural history and the consecutive post-therapeutic evolution principally depend upon the evolutionary stage and the cytomorphologic features of the lesions [1, 2].
Insular thyroid carcinoma (ITC) is a rare mentioned but aggressive subtype of thyroid neoplasm situated in an intermediate position between the highly differentiated follicular and papillary carcinomas with good prognosis and the undifferentiated (anaplastic) cancers which are almost uniformly lethal. Actually ITC is framed together with trabecular and solid tumors in the poorly differentiated subgroup of thyroid cancers representing 4-6% of all the malignant lesions of this gland. The lesion is characterized by accelerated tumoral growth and extra nodal invasion, precocious nodal and distant metastasis, frequent recurrences and an overall mortality of 60-85% [3].
An uncommon case of ITC with precessive lateral cervical lymphadenopathy (pTxN2M0), with presence of follicular features at FNAB, and also with a 5 mm 0 impalpable thyroid nodule discovered intraoperatively, all of them leading to the diagnosis, is presented.
Case report
CC a 59-year old male whose chief complaint is a huge right cervical conglomerate of lymph nodes appearing one year ago. Personal patient’s history revealed a nodal biopsy on the same side performed 15 years ago but with benign histology. Physical examination revealed a right sided 3x5 cm firm, painless but fixed conglomerate of lymph nodes. (Fig 1) There is no significant another adenopathy and the thyroid gland is not papable. The general clinical condition is good without evidence of any physical or metabolic and endocrine disturbances. Excepting a discrete anemia (Ht= 39%), the standard laboratory tests including TSH and T4 measurements were in normal range. Cervical ultrasonography demonstrate a hypo echoic huge irregular mass including multiple more or less distinct solid, heterogeneous images of 1-2 cm in diameter, related to the middle and inferior cervical node groups. The thyroid gland is also heterogeneous but with normal contour and dimensions (Fig 2). Thyroid scan reveals similar characters but without cold areas.

Figure 1: Right laterocervical adenopathy.
Fine needle aspiration biopsy showed aggregates of monomorphic, isolated, clustered or overlapping cells with indistinct limits, reduced cytoplasm, round nuclei and rare mitosis, situated in a scanty fibrovascular stroma leading to the diagnosis of follicular thyroid carcinoma metastasis.
Figure 2: Ultrasound of the lateral cervical adenopathy.
During operation a selective neck lymph node dissection with en block extirpation of the IInd and IVth node groups along with internal jugular vein, accessory spinal nerve and sternocleidomastoid muscle conservation was done.
The thyroid had a normal volume with discrete lobulated surface but a firm, unencapsulated, grey-white solitary centrolobar nodule of 5 mm 0 was discovered at palpation. After the main vascular pedicles interception a standard total thyroidectomy was done. The entire surgical specimen weighted 60 g.
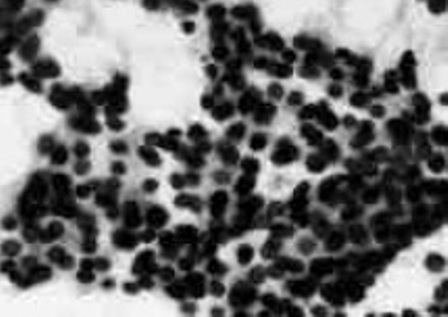
Figure 3: ITC: FNAB aspect.
Microscopic sections both in the lateral cervical nodes and in the thyroid nodule showed the presence of round and oval “monotonous” cells, with scant cytoplasm and regular nuclei with granular chromatin and mitosis, disposed in follicles and “insular groups” separated by fibrovascular stroma (Fig 4). Intravascular tumor emboli and necrotic areas were also identified. The rest of the gland showed inequal colloid-filled or atrophic areas, thyroid follicles, stromal reaction, hyalinization, hemorrhagic zones but without another neoplasic focus. Final diagnosis: insular thyroid carcinoma.
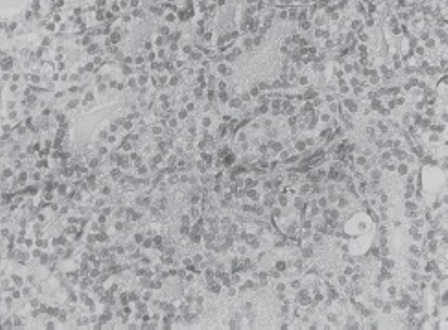
Figure 4: ITC: Histologic characteristic aspect low magnification.
Immediate postoperative course was uneventful and after 5 weeks rh TSH (recombinant human thyrotropin) was administered and a dose of 100 mCi 131 I was given. Afterwards the patient received thyroid hormone suppressive therapy. Periodic clinical, biological and imaging follow-up at 1-3 years did not reveal recurrences or metastasis.
Discussion
Initially described by Langhans (1907) as “wuchernde struma” (proliferative struma) and later by Sakamoto (1983) as a poorly differentiated thyroid carcinomatous entity, the insular thyroid carcinoma is defined by Carcangiu (1984) as a distinct variant of glandular neoplasia characterised by presence of solid nests –“insulae” of little uniform tumoral cells, constituting a variable number of follicles containing thyroglobulin [4, 5, 6].
Adjoining moderate mitotic activity, capsular and vascular invasion and necrotic foci made together a peritheliomatous structure. Later ITC was added assimilated to the “aggressive” thyroid carcinomas of follicular origin subgroup which includes trabecular, insular and solid lesions with an intermediary behavior and prognosis between epithelial (papillary and folliculary) and anaplasic (undifferentiated) cancers. Some authors underlined the peculiarities of the insular lesions i.e. intense cellularity, presence of atypias, increased number of mitosis and necrotic areas but especially of the accelerate rhythm of growth while other experts also included the columnar and tall cell cancers in this subgroup [7, 8].
From the clinical point of view the majority of these tumors developed as solitary or multiple thyroid nodules, sometimes on a preexistent goiter, having a female predominance: report F/M ♀/♂ - 2.1-2.5/1 and a mean age of 50-55 years. ITC have a rapid invasive evolution both locally reaching great dimensions (5-10 cm) and causing compressive complications such as dysphonia or Horner syndrome and also extra thyroid metastasis of the lymph nodes or distant sites (lung, bone, liver), present in 20% of cases at the initial examination putting them in high-risk lesions in accordance with De Groot, AMES or MACIS scores at the moment of diagnosis [9, 10, 11]. Medium and genetic factors as gene p53 (the molecular marker of the tumor dedifferentiation) mutations, detected in 20-30% of cases and also N-RAS and BRAF genes alterations can be incriminated for their prognostic stratification [12]. The diagnosis of ITC can be difficult, the gold standard being histology completed by ancillary studies as immunohistochemistry and molecular techniques [3, 9].
FNAB is delusive evidencing subtle modifications leading often to a erroneous initial diagnosis of follicular carcinoma as in our observation. Cellular smears composed of monomorphic groups of small cells disposed in large layers or clusters, with high N/C ratio, frequent mitotic activity and presence of necrotic or cellular debris are suggestive for ITC [13, 14].
Ultrasound is a descriptive but not a diagnostic investigation for assessing the dimensions and tumoral invasion, the eventual adenopathy (as in our observation), the often predominant hypoechogeous, solid/homogeneous aspect, but the absence of halo or cystic modifications. Ultrasound is also used to guide needle biopsy [3, 4].
131 I scintigraphy (radioiodine uptake is increased in 75% of ITC), Tc-99 MIBI (methoxisobutilisonitril) or DMSA (acid dimercaptosuccinic) showed evidence of the hypofixant or cold areas and established the macroscopic tumoral features remaining important in the diagnosis of metastatic deposits and in the post therapeutic monitoring. I-131 whole-body scan remains a prime line investigation [15, 16].
Computer tomography and magnetic resonance imaging defines the volume but especially the limits and extension of the tumor (mediastinum, thorax).
18F-Fluorodeoxiglucose positron emission tomography (FDG-PET) only or associated with CT or/and magnetic resonance imaging, reveals the prevailing character of the less differentiated lesions positive to FDG, being useful in precise preoperative staging and post therapeutic restaging and follow-up of the ITC [5, 15].
Indirect laryngoscopy and fiber optic examination of the upper respiratory tract are mandatory to discover the eventually RN involvement (present in 25% of cases) or local invasion. However the great majority of these tumors are diagnosed only by postoperative paraffin examination.
The consensus meeting held in Turin (2006) proposed uniform criteria for the diagnostic of ITC:
- Solid, trabecular or “insular” growth pattern;
- The absence of papillary carcinoma’s characteristics;
- Existence of “complicated” nuclei with greater mitotic activity and tumoral necrosis [2].
- The rest of microscopic description traced with the one of our observation.
- Electronic microscopy showed an abundant cytoplasm, endoplasmic reticulum, numerous mitochondria and free ribosoms [13, 14, 15].
We must also remember the “focal” ITC coexisting with zones of papillary, folliculary or even anaplastic carcinoma and also the dedifferentiation of some initially less aggressive lesions. The mainstay ITC therapy is total thyroidectomy with lateral and even central neck dissection followed by high dose radioiodine administration, efficacious in distant metastases present in 1/3 of observations [16, 17, 18].
Conclusion
Our case exemplify a rare described epithelial thyroid tumor (about 200 cases in literature) characterized by intermediate aggressiveness between the cancers of follicular origin and anaplastic tumors, advanced stage at presentation with a rapidly invasive behavior, accelerated local and general dissemination, local recurrences and poor prognosis. Atypical debut with precessive adenopathy, lacking goiter and the presence of follicular features at the FNAB, determined diagnosis difficulties, this one been established only after the paraffin examination. Radical surgical treatment completed by radioiodine ablation can assure however the cure or prolonged survival in a variable percentage of cases as in our patient.
Author’s Contribution
MRD: Conceived and drafted the manuscript, performed surgery.
MG: Literature research.
MG: Pathology.
SD: Essentially involved in manuscript drafting.
Conflict of Interest
There is not personal and financial conflict of interest.
Ethical considerations
The authors obtained the patient's consent foe the publication on this paper.
References
[1]. Flynn SD, Formann BH, Stewart AF, Kinder BK: Poorly differentiated (“insular”) carcinoma of the thyroid gland: an aggressive subset of differentiated thyroid neoplasms. Surgery 1988, 104(6): 963-70. [Pubmed].
[2]. Sobrino-Simoes M Arbores-Saavedra J, Tallini G, Santoro M, Volante M, Pilotti S et al: “Poorly differentiated carcinoma”. In World Health Organisation Classification of Turin. Pathology and Genetics of Tumours of Endocrine Organs. DeLellis RA, Lloyd RV, Heitz PU, Eng C (eds), Lyon IARC Press 2004: 73-6.
[3]. Kazaure HS, Roman SA, Sosa JA: Insular thyroid carcinoma: a population-level ana-lysis of patient characteristics and predictors of survival. Cancer 2012, 118(13): 3260-7. [Pubmed].
[4]. Langhans T: Uber des epithelialen form der malignen struma. Virchows Arch 1907, 189: 69-188.
[5]. Sakamoto A, Kasai N, Sugano H: Poorly differentiated carcinoma of the thyroid: a clinico-pathologic entity for a high risk group of papillary and follicular carcinoma. Cancer 1983, 52(10): 1849-55. [Pubmed].
[6]. Carcangiu ML, Zampi G, Rosai J: Poorly differentiated (“insular”) thyroid carcino-ma: a reinterpretation of Langhans’ “wuchernde struma”. Am J Surg Pathol 1984, 8(9): 655-8. [Pubmed].
[7]. Paik SS, Kim WS, Hong EK, Park MH, Lee JD: Poorly differentiated (“insular”) carci-noma of the thyroid gland – two cases report. J Korean Med Sci 1997, 12(1): 70-4. [Pubmed].
[8]. Hiltzik D, Carlson DL, Tuttle R, Chuai S, Ishill N, Shaha A et al: Poorly differentia-ted thyroid carcinoma defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer 2006, 106(6): 1286-95. [Pubmed].
[9]. Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Kotoh R et al: Poorly differentiated thyroid carcinoma: The Turin proposal for the use of uniform diagno-stic criteria and an algorithm diagnostic approach. Am J Surg Pathol 2007,(8): 1256-64. [Pubmed].
[10]. Sadow PM, Faquin WC: Poorly Differentiated Thyroid Carcinoma: An Incubated Entity. Frontieres Endocrinol (Laussane): 2012, 3(77): 1-4. [Pubmed].
[11]. Kini H, Nirupama M, Rau AL, Gupta S, Augustine A: Poorly differentiated (insular) carcinoma arising in a long-standing goitre: A cytologic dilemma. J Cytol 2012, 29(1): 97-9. [Pubmed].
[12]. Soares P, Lima J, Preto A, Castro P, Vinagre J, Celestino R et al: Genetic alterati-ons in poorly differentiated and undifferentiated thyroid carcinomas. Curr Genomics 2011, 12(8): 609-17. [Pubmed].
[13]. Pietribiasi F, Sappino A, Papotti M, Bussolati G: Cytologic features of poorly diffe rentiated “insular” carcinoma of the thyroid as revealed by fine-needle aspiration bi-opsy. Am J Clin Pathol 1990, 94(6): 687-92. [Pubmed].
[14]. Bongiovanni M, Bloom L, Krane JF, Baloch ZW, Powers CN, Hintermann S et al: Cyto-morphological features of poorly differentiated thyroid carcinoma.A multi-insti-tutional analysis of 40 cases. Cancer 2009, 117(3): 185-94. [Pubmed].
[15]. Fat I, Kulaga M, Dodis R, Carling T, Teoharis C, Rennert NJ: Insular variant of poorly differentiated thyroid carcinoma. Endocr Pract 2011, 17(1): 115-21. [Pubmed].
[16]. Agha A, Glockzin G, Woenckhaus M, Dietymaier V, Iesalnicks I, Schlitt HJ: Insular carcinomas of the thyroid exhibit poor prognosis and long-term survival in compara-sion to follicular and papillary T4 carcinomas. Langenbecks Arch Surg2007, 392(6): 671-7. [Pubmed].
[17]. Cornetta AJ, Burchard AE, Pribstkin EA, O’Reilly RC, Palazzo JP, Keane WM: Insu-lar carcinoma of the thyroid. Ear Nose Throat J 2003, 82(5): 384-6. [Pubmed].
[18]. Sironen P, Hagstrom J, Maenpaa H, Louhimo H, Heikkila A, Heiskanen A et al: Anaplastic and poorly differentiated thyroid carcinoma: therapeutic strategy and treatment outcome of 52 consecutive patients. Oncology 2010, 79(5-6): 400-8. [Pubmed].

