Case Report

Bilateral Angiosarcoma of Breast
1Seema Khanna, 1Shashi Mishra, 1Chandan Jha, 2Satendra Kumar, 1Sanjeev K Gupta
1Department of Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
2Department of Dental Sciences, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
-
Submitted: Tuesday, July 09, 2013
-
Accepted: Thursday, August 15, 2013
-
Published: Friday, October 18, 2013
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Introduction
Sarcoma of the breast is a rare tumor with poor prognosis. Majority of the cases described in literature are secondary to radiotherapy. Fine needle aspiration cytology is a good tool for diagnosis. Optimal treatment for breast sarcoma is still unclear.
Case Report
A 25 years female presented with a recurrent 10x8 cm lump in her left breast. Systematic examination of the contralateral breast revealed a 4 x3 cm lump in her right breast also. FNAC suggested the possibility of a malignant mesenchymal lesion. Bilateral simple mastectomy was performed. Histopathology revealed bilateral angiosarcoma of the breast with extensive haemorrhage and necrosis. Immunohistochemistry was positive for Vimentin and CD 31 but negative for S 100. Adjuvant chemotherapy (MAID regime) was given to the patient.
Discussion
Primary sarcoma of the breast usually presents as a lump with variegated consistency in the breast in a young female often secondary to radiotherapy. FNAC is diagnostic. Angiosarcoma is the most common variant. 5 year survival ranges from 40%-91 %. Margins of surgical resection are an important prognostic indicator. Axillary dissection is generally thought to be unnecessary for soft tissue sarcomas.
Conclusion
Adequate surgical excision, grade and tumor diameter seem to be the most important prognostic factors.
Key Words
Sarcoma, Breast, Mastectomy, chemotherapy
Introduction
Primary breast sarcoma was first described in 1887 and occurs in less than 1% of women with breast malignancy [1].
Only a few hundred cases have been reported in literature and most of them are secondary to radiotherapy [2]. Fine needle aspiration cytology (FNAC) is a good tool for diagnosis. The entity, which arises from breast mesenchyme, includes angiosarcoma, malignant fibrous histiocytoma, stromal sarcoma, liposarcoma, leiomyosarcoma, dermatofibrosarcoma protuberans, osteosarcoma, fibrosarcoma and rhabdomyosarcoma[8]. The optimal treatment for breast sarcoma is still unclear. There is no consensus on the extent of surgery and the need for adjuvant chemo-radiation. Overall, the prognosis of breast sarcoma is poor. Tumor grade and size of the lesion influence prognosis. Early diagnosis and treatment impact survival. We here report a case of bilateral primary angiosarcoma of the breast.
Case Report
AA 25 years female presented with lump in left breast for one year. She had undergone lumpectomy for the same pathology one year back elsewhere but the lump reappeared after two months of operation. The histopathology of the excised lump was not available. On examination, lump was 10x8 cm size with variegated consistency, non tender and mobile with non tender, mobile lymph nodes in left axilla. On examination of the contralateral breast, we noticed a 4x3 cm lump in right breast which was firm, mobile, non tender along with few subcentric lymph nodes in right axilla. FNAC suggested the possibility of a malignant mesenchymal lesion on left side and that of right breast lump suggested features of spindle cell sarcoma. Hence, with the diagnosis of primary sarcoma of the bilateral breasts, we performed bilateral simple mastectomy. Post operative histopathology was angiosarcoma with extensive haemorrhage and necrosis on both sides (Figure 1). Margins on both the sides were free from tumor.
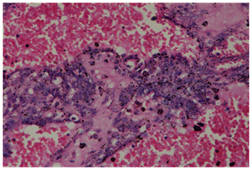
Figure 1: Microphotograph (400X) showing Angiosarcoma breast with arborizing vascular channels in pool of blood
On immunohistochemistry, tumors were strongly positive for Vimentin and CD 31 and negative for S-100 (Figure 2). Postoperative course was uneventful. The patient was put on postoperative chemotherapy (MAID regimen). Adjuvant radiotherapy was planned after completion of chemotherapy.
Discussion
Sarcomas occur anywhere in the body with preponderance for extremities. Intra-abdominal sarcomas are fairly common and may be visceral or retroperitoneal [3]. Primary sarcoma of breast is rare entity. Common presentation is breast lump in young females in second to fourth decade of their life. FNAC is a good tool to diagnose breast sarcoma [4]. The reported 5-year survival rates range from 40 to 91% [5, 6]. Zelek et al [1] showed higher rates, however, 57/83 women in their series had malignant fibrous histiocytoma. Pandey et al [7] found 39% overall 3-year disease-free survival (DFS).
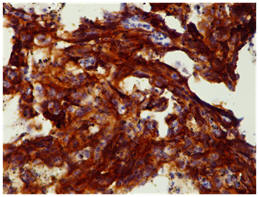
Figure 2: Microphotograph of Angiosarcoma breast CD31 positive (brown Stain)
Complete microscopic resection of the primary tumor is an important factor for local disease control, overall survival (OS), and DFS [5, 7]. Pandey et al. [7] claimed the margin of surgical resection to be the most important prognostic indicator. They also found 33.3% 3-year survival in patients with negative margins, 57% in patients where margins were not known, and 0% in patients with positive margins (p = 0.05). However, in the retrospective analysis by Zelek et al [1], the 10-year OS and DFS rates were 62 and 50%, respectively. For tumors measuring less than 5 cm, 5–10 cm, and more than 10 cm, the 10-year OS and DFS rates were 76 and 66%, 68 and 55%, and 28 and 15%, respectively (p = 0.002 and 0.009, respectively). In the multivariate analysis, the tumor size and histological grade were correlated with the 10-year DFS rate (p = 0.04 and 0.01, respectively), but only the histological grade was correlated with OS (p = 0.01). Mastectomy was not found to be necessary to improve DFS in the report by Blanchard et al. [8], and wide local excision yielded equally good local control. Axillary dissection is thought to be unnecessary for most soft tissue sarcomas, since these tumors rarely metastasize via the lymphatics. Our patient was diagnosed preoperatively on basis of FNAC and underwent bilateral mastectomy. Radiation is stated to be efficacious after surgical resection, particularly with microscopically involved margins; however, this was not supported by other studies such as that of Blanchard et al. [8]. Kirova et al. [9] found that most of the examined cases of radiotherapy-induced sarcomas were angiosarcomas located in the primarily radiated areas.
Immunohistochemistry (IHC) may be useful to rule out other tumors such as non-mesenchymal malignant tumors or sarcomas with a specific line of differentiation. Desmin, vimentin, smooth muscle antigen, keratin and leukocyte common antigen, CD34, HMB45, EMA, and S-100 should all be analyzed in these sarcoma patients. In our patient, IHC analysis only vimentin and CD 31 positivity was detected with no specific cell markers for subtypes of primary breast sarcoma.
Conclusion
Most invasive breast neoplasms are epithelial tumors and mesenchymal breast tumors are rarely seen. The sarcomas of breast may at times be bilateral with similar or different histology on both the sides. FNAC can easily diagnose these tumors and surgery is the mainstay of treatment. In primary breast sarcomas, adequate surgical excision of the tumor, grade, and tumor diameter seem to be the most important prognostic factors.
Competing Interests
None
Acknowledgements
None
Ethical Considerations
Written Informed consent was obtained from the patient for publication of
this case report.
References
1]].Zelek L, Llombart-Cussac A, Terrier P, Pivot X, Guinebretiere JM, Le Pechoux C, Tursz T, Rochard F, Spielmann M, Le Cesne A: Prognostic factors in primary breast sarcomas: a series of patients with long-term follow-up. J Clin Oncol 2003; 21:2583–2588.[pubmed]
2]][Moore MP, Kinne DW: Breast sarcoma. Surg Clin North Am 1996; 76:383-392.[pubmed]
3]Wood WC, Muss HB, Solin LJ, Olopade OI: Malignant tumors of the breast; in De Vita VT, Hellman S, Rosenberg SA (eds): Cancer Principles and Practice of Oncology, ed 7. Philadelphia, Lippincott-Williams and Wilkins, 2005, pp. 1415–1477."
4]]Pai MR, Upadhyaya K, Naik R, Malhotra S. Bilateral Angiosarcoma Breast diagnosed by Fine Needle Aspiration Cytology. Indian Journal of Pathology and Microbiology 2008; 51(3):421-3.[pubmed]
5]]Pollard SG, Marks PV, Temple LN, Thompson HH: Breast sarcoma: a clinicopathologic review of 25 cases. Cancer 1990; 66:941–944.[pubmed]
6]]Callery CD, Rosen PP, Kinne DW: Sarcoma of the breast: study of 32 patients with reappraisal of classification and therapy. Ann Surg 1985; 201:527–532.[pubmed]
7]]Pandey M, Mathew A, Abraham EK, Rajan B: Primary Sarcoma of the Breast. J Surg Oncol 2004; 87: 121–125.[pubmed]
8]]Blanchard DK, Reynolds CA, Grant CS, Donohue JH: Primary nonphylloides breast sarcomas. Am J Surg 2003; 186:359–361.[pubmed]
9]]Kirova YM, Vilcoq JR, Asselain B, Sastre-Garau X, Fourquet A: Radiation-induced sarcomas after radiotherapy for breast carcinoma: a large-scale single-institution review. Cancer 2005; 104:856–63.[pubmed]

