Case Report

Sertoli Leydig cell tumor with unusual tumor marker: management dilemmas
Sreelakshmi Kodandapani, Jayaram Nambiar, Vidya Kamath, Muralidhar V Pai and Lakshmi Rao
Department of Obstretics and Gynaecology, Kasturba Medical College, Manipal 576104, India
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Submitted: March 25, 2013; Accepted: May 23, 2013;Published: July 26, 2013
Abstract
Introduction
Sertoli Leydig Cell tumors are rare sex-cord tumors. They cause virilization of female characterized by hirsuitism, temporal baldness, hoarseness of voice and clitoromegaly. Rarely they have heterologous cells and produce alpha fetoprotein (AFP). They cause raised serum alphafetoprotein which can be detected in tissue by immunohistochemistry.
Case Presentation
We report an 18 year old girl who presented to us with hoarseness of voice, hirsuitism, pain abdomen and vomiting. Huge ovarian mass was diagnosed by ultrasound. She had very high serum alphafetoprotein levels. She underwent staging laparotomy with salpingo-oophorectomy. Histopathology was Sertoli Leydig Cell Tumor with hepatoid differentiation. Postoperatively she received chemotherapy. Serum alphafetoprotein was monitored and levels reduced, thus indicating response to treatment.
Conclusion
Incidence of Sex cord tumors per se is rare. Hepatoid differentiation of these tumors with production of alphafetoprotein is very rare. Although five similar cases are reported earlier, we believe addition of this case is useful. Detailed preoperative evaluation and histopathology were useful in this case management
Keywords
Sertoli leydig cell tumor, alphafetoprotein hirsuitism, immunohistochemistry
Introduction
Sertoli Leydig Cell tumors, also known as arrhenoblastomas are rare sex cord cell tumors comprising of less than 0.5% of ovarian tumors [1]. They are usually unilateral and consist of testicular elements. Hence patient presents with varying degrees of virilization. Seventy five percent of these tumors present in second and third decade of life. They can have varying degrees of differentiation with twenty percent having heterologous elements. Heterologous elements can be endodermal structures namely gastrointestinal organs like intestines, bone, cartilage, muscle [2]. Rarely hepatoid differentiation producing alpha fetoprotein (AFP) is reported [3]. In general, AFP is produced by yolk sac tumor, occasionally by teratoma and dysgerminoma. We report a case of hepatoid differentiation in a young girl producing high quantities of alphafetoprotein and whose detection helped in appropriate case management.
Case presentation
We report a case of an 18 year old unmarried girl who presented to us as hoarseness of voice and excessive hair growth since 3 years and pain abdomen with vomiting since ten days. She had attained menarche at the age of 13 years with regular menses and normal flow. Hoarseness of voice and excessive hair growth was progressive since 3 years. Pain abdomen was acute in onset in lower abdomen with vomiting. Pain aggravated with walking and at work but minimally relieved with rest. She was not tolerating any solids or fluids at the time of admission. She did not have history of loss of weight or appetite. She did not have bowel and bladder disturbances. She did not have any other significant medical illness and there was no significant family history. She had transabdominal ultrasound at a private hospital which showed a huge ovarian mass. Hence she was subsequently referred to our teaching hospital. On examination, she had stable vital signs with no signs of dehydration. She had extensive hair growth over Upper lip, chin, chest, upper back, lower back, upper abdomen, lower abdomen, upper arms, forearms and thighs. Thus her Ferriman Gallwey score was 36 with extensive hair growth over the body [4].
At abdominal examination, huge ovarian mass of variegated consistency was palpable occupying right iliac fossa, hypogastrium and upto umbilicus. It was approximately 20X15 cm. There was free fluid in the abdomen. Mass was tender on deep palpation. There was restricted mobility and lower border was not made out. Liver and spleen were not palpable. Mild clitoromegaly was noted at local examination. Vaginal examination was denied as patient was not sexually active. Her hemoglobin was 11g%, with normal liver function, renal function, electrolytes reports. Chest radiograph and electrocardiogram was normal. Hormonal profile revealed raised, serum testosterone (1.5ng/ml; normal: 0.2-1.2ng/ml) and alphafetoprotein was 830 ng/ml (normal <1ng/ml). However CA125, DHEAS and LDH were normal. At transabdominal ultrasound, huge ovarian mass 18X15 cm with cystic and solid areas. Figure 1 is ultrasound picture showing mass of 20X15 cm with solid and cystic areas. Uterus was normal. Other ovary was not visualized. There was no free fluid. Liver, spleen, kidneys, urinary bladder and intestines were normal. Doppler ultrasound of tumor was inconclusive. Based on these findings, possibility of Sertoli Leydig Cell tumor was thought of. However we could not explain raised AFP.
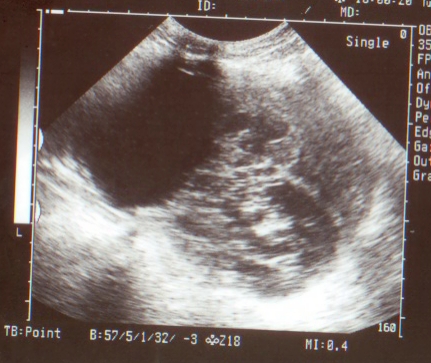
Figure 1: Transabdominal ultrasound showing ovarian tumor 15X10 cm with solid and cystic components
She was counseled for laparotomy, remote possibility of major organ injuries and inevitable removal of ovaries and uterus as the last resort was also explained. Abdomen was opened by midline vertical incision. There was no ascitic fluid; hence peritoneal washings were taken for malignant cells. Right ovary was enlarged to 20X 15 cm with solid and cystic areas with one and half twist at the pedicle, thus torsion was the cause for her recent onset pain abdomen and vomiting. Capsule was intact and smooth. There were no adhesions. There was no spillage of tumor cells during surgery. The abdominal cavity was explored systematically but there were no deposits anywhere else in the cavity. The para-aortic lymph nodes were not enlarged. Other ovary, uterus and omentum were normal. Right salpingoophorectomy was done. Figure 2 is huge ovarian mass with evidence of hemorrhage and necrosis. She had uneventful postoperative period and was discharged on 7th postoperative day.

Figure2: Huge ovarian mass with hemorrhage and necrosis due to torsion
Macroscopically, oophorectomy specimen weighed 1.6kg and measured 14x12x9 cm. Tumor appeared cystically enlarged and congested. On cut section, there were multilobular cysts filled with few serosanguinous fluid. The wall of the cyst appears thickened and showed solid areas, grey white, yellow and hemorrhagic areas.
At histopathology, tumor showed a solid and cystic tumor with solid areas composing of 2 types of cells. Predominant cell type is oval to columnar shaped cells with oval vesicular nucleus, anisonucleosis, inconspicuous nucleoli arranged in cords, sheets & clusters. Many of the cells have abundant foamy cytoplasm. Sheets of cells with abundant eosinophilic cytoplasm, intracytoplasmic and extracellular PAS positive diastase resistant hyaline globules, few were along nodules and showed marked nuclear atypia & bizarre nuclei (degenerating clusters) spindle cell stroma, some of which appear very primitive leydig cells, hemosiderin laden macrophages. Cystic component is composed of cystic structures of variable size lined by small cells described as above & hobnail, flattened cells forming a reticular pattern. Foci of hepatoid differentiation characterized by large polygonal cells with intracytoplasmic bile, vesicular nucleus & prominent nucleoli were seen. No Schuller Duval bodies noted. Capsule was intact. Tube was free. Peritoneal washings were negative for malignant cells. Tumor cells were inhibin positive and Cytokeratin (CK) 7, CK20 and CK117 - negative. These tumor markers are used to rule out metastasis from gastrointestinal tract. Figure 3 is the photomicrograph of the tumor with inhibin positive hepatocytes. Histopathological diagnosis was Sertoli Leydig Cell tumor with intermediate and hepatoid differentiation and torsion.

Figure 3: Histopathology of tumor showing hepatocytes
Based on all findings, final diagnosis of Stage IA Sertoli Leydig cell tumor with intermediate and hepatoid differentiation was made. She received four cycles of Bleomycin, Etoposide and Cisplatin regimen in 3 months time. Serum AFP levels were measured at follow up, which showed drastic fall after treatment. Figure 4 shows the fall of AFP level during her treatment. Serum testosterone was normal. Her virilization signs have reduced in 6 months follow up. There was no further growth of hair. She had regular menstruation.
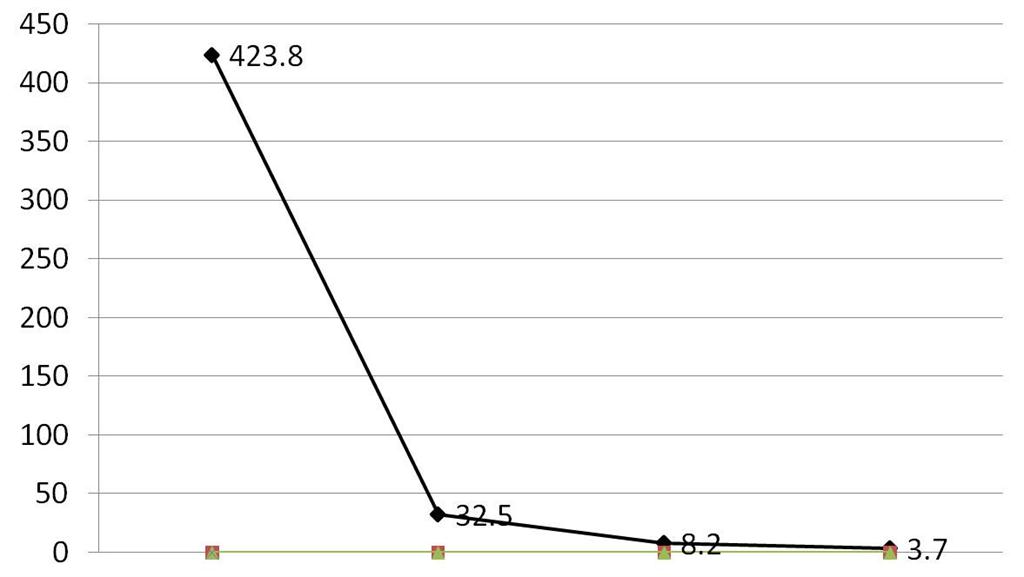
Figure 4: Graph showing levels of alphafetoprotein (ng/ml) postoperatively
First value: Preoperative value Second value: 7th post-operative day Third value: at the end of chemotherapy Fourth value: at the end of 6 months
Discussion
We here present an unusual case of SLCT with heterologous elements. Heterologous elements are observed in approximately 20% of SLCT. These can be further separated into 2 basic types: endodermal elements and mesenchymal elements. The SLCT with heterologous mesenchymal elements are usually poorly differentiated in contrast to neoplasms with endodermal elements, which typically are of intermediate differentiation [5]. The majority of these patients are seen during the second and third decades of life, with the average age at diagnosis of 25 years. Progressive defeminization was the clinical presentation in approximately 50% of cases as seen in this patient.
The most important prognostic factors in these tumors are: degree of differentiation and the stage of presentation. In a review of 207 cases by Young and Scully (1985) [1] all well-differentiated tumors were benign, whereas 11% of tumors with intermediate differentiation, 59% of tumors with poor differentiation, and 19% of those with heterologous elements were malignant. In another study of 64 patients who had intermediate or poorly differentiated SLCT, a survival rate of 92% was noted at both 5 and 10 years [6]. CK7, CK20 and CK117 immunestains were done to differentiate primary ovarian tumor from metastatic tumors. They were negative in this patient’s tumor.
Adjuvant chemotherapy is considered for patients who have poor prognostic factors. The malignancy rate in tumors with heterologous elements is 15% to 20% [1]. Adjuvant chemotherapy in stage I is given to those patients who have poorly differentiated SLCT or SLCT with heterologous elements or a metastatic tumor of any histologic type [7]. Patient in this report received adjuvant chemotherapy for SLCT in stage I. Two factors considered were incomplete staging of the tumor and the presence of heterologous elements on histopathologic study of the tumor. The following chemotherapy regimens were considered:
1. Cisplatin, doxorubicin, cyclophosphamide (PAC);[8] 2.Vincristine,actinomycinD,cyclophosphamie (VAC);[9] and 3. Bleomycin, etoposide, and cisplatin (BEP) [10]
The BEP regimen is a comparatively safe chemotherapeutic regimen because it does not affect the fertility status of the patient [10].
In conclusion, SLCT is a rare ovarian sex-cord tumor that usually occurs unilaterally. SLCT should always be considered in a young female patient who has symptoms of virilization and an ovarian mass on examination or investigation.
Management issues mostly revolve around the histopathology of the tumor. Poorly differentiated tumors require aggressive management because the chances of them being malignant are high. Intermediately differentiated tumors need an individualized approach.
Authors’ Contributions
SKN: Literature search and preparation of manuscript
JN: Literature search and preparation of manuscriptMVP: Literature search and preparation of manuscriptVK: Literature search and preparation of manuscriptLR: Literature search and preparation of manuscript
Ethical Considerations
Written consent was obtained from the patient for publication of this case report.
Conflict of Interests
The authors declare that there are no conflicts of interest.
Acknowledgement
None
Funding
None declared
[1].Young RH, Scully RE. Ovarian Sertoli-Leydig cell tumours. A clinicopathological analysis of 207 cases. Am J Surg Pathol. 1985; 9:543–569 [pubmed]
[2].Mathur SR, Bhatia N, Rao IS, Singh MK. Sertoli Leydig cell tumour with heterologous gastrointestinal epithelium: a case report. Indian J Pathol Microbiol. 2003; 46:91–93 [pubmed]
[3].Talerman A, Haije WG, Bagerman L. Serum AFP in patients with germ cell tumors of the gonads and extragonadal sites: Correlation between endodermal sinus (yolk sac) tumor and raised AFP. Cancer. 1980;46:380-385 [pubmed]"
[4].Ferriman D, Gallwey JD: Clinical assessment of body hair growth in women. Journal of Clinical Endocrinology 1961; 21:1440–1447.[pubmed]
[5].Lantzsch T, Stoerer S, Lawrenz K, Buchmann J, Strauss H-G, Koelbl H. Sertoli-Leydig cell tumour. Arch Gynecol Obstet. 2001;264: 206–208 [pubmed]
[6].Zaloudek C, Norris H. Sertoli-Leydig cell tumors of the ovary. A clinicopathological study of 64 intermediate and poorly differentiated neoplasms. Am J Surg Pathol. 1984;8: 405–418 [pubmed]
[7].Sood AK, Gershenson DM. Management of early-stage ovarian cancer. In: Bristow RE, Karlan BY, editors. Surgery for Ovarian Cancer: Principles and Practice. London, UK: Taylor and Francis; 2005. pp. 57–86.
[8].Gershenson DM, Copeland LJ, Kavanagh JJ, et al. Treatment of metastatic tumour of the ovary with cisplastin, doxorubicin and cyclophosphamide. Obstet Gynecol. 1987;70:765–769. [pubmed]
[9].Schwartz PE, Smith JP. Treatment of ovarian stromal tumours. Am J Obstet Gynecol. 1976;125:402–411.[pubmed]
[10].Gershenson DM, Morris M, Burke TW, et al. Treatment of poor-prognosis sex cord-stromal tumors of the ovary with the combination of bleomycin, etoposide, and cisplatin. Obstet Gynecol. 1996;87:527–531[pubmed]

