Case Report

Anesthetic management of a child with type І homocystinuria
1Hamid Zahedi, 1Mahshid Nikooseresht, 2Mohamad Ali Seifarabie, 3Koroush Sheibani
- 1Department of Anesthesiology and intensive care. Besat Hospital, Hamadan University of Medical Sciences, Resalat Boulevard, Hamadan, Iran.
- 2Department of Social Medicine, Hamadan University of Medical Sciences, Shahid Fahmide Street, apposite Mardom Park. Hamadan, Iran
- 3Clinical Research and Development Center, Imam Hossein Medical Center, Shahid Beheshti University of Medical Sciences, Shahid Madani Street,Tehran, Iran
- Submitted: December 22, 2012;
- Accepted January 06, 2012
- Published: January 06, 2012
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Homocystinuria is a rare autosomal recessive disease with multiple systemic manifestations, which is classified into three types (type's Ι-III). Here we report an 8-year-old boy who required anesthetic care during an ocular surgery including lensectomy under general anesthesia. Proper precautions should be taken during anesthetic management of such a patient since some particular Anesthetic complications like preoperative thromboembolism and hypoglycemia may worsen the course of Anesthesia. We provided successful anesthetic management for our patient and he was discharged without subsequent complications.
Introduction
Homocystinuria is a rare autosomal recessive disease with an incidence of 1:200,000 live births. Three major types of homocystinuria have been identified caused by three different defected enzymes associated with the metabolism of amino acid methionine (Fig 1) [1,2].
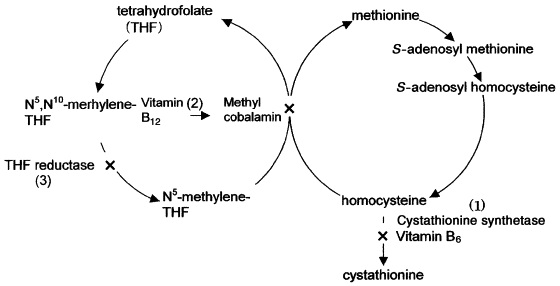
Fig. 1. Metabolic pathway of methionine. Homocystinuria type I is due to a deficiency of the cofactor pyridoxine (vitamin B6) and the enzyme cystathionine synthase (1), which synthesizes cystathionine from homocysteine and serine. Type II is due to a deficiency of the enzyme tetrahydrofolate methyltransferase (2), and type III is due to a defect of the enzyme tetrahydrofolate reductase (3); both of which convert homocysteine to methionine
Type Ι (Classic homocystinuria) is caused by a deficiency of cofactor pyridoxine (vitamin B6) and the enzyme cysthatione-β-synthase. Type II is due to a deficiency of the enzyme tetrahydrofolate methyltransferase and type III is caused by a defect of the enzyme tetrahydrofolate reductase. More than 95% of patients with homocystinuria are type Ι [1].
Although this disorder is rare, surgical interventions and anaesthesia care is frequently required for ophthalmologic (ectopia lentis) or skeletal (pectus excavatum, scoliosis) manifestations [3,4]. Here we report a case of classic homocystinuria (type Ι) and anaesthetic management of such a patient.
Case report
An 8 year old boy (American Society of Anaesthesiologists physical status Π, 127 cm height, 25 kg weight) presented with the complaint of diminished vision and was scheduled for elective lensectomy under general anaesthesia. His past medical history was unremarkable for anaesthesia and surgery. He was the first child of his parents who had a family marriage (between cousins). The diagnosis of type І homocystinuria was made three ago, during a workup for the cause of developmental delay and diminished vision. High levels of plasma methionine and homocysteine were detected and the patient was put on oral folic acid (1mg once per day), and pyridoxine (100mg three times per day).
The child was admitted to the hospital the day before surgery. Homocysteine and methionine levels were in normal range, other preoperative laboratory data was as below:
Hemoglobine=12.6 g/dl, Platelets=401,000/µl (150,000-450,000), Bleeding Time=3 min (control=1-4min), Clotting Time=7min (control=1-7.5 min), Prothrombin Time=13sec (control=12s), Activated Prothrombin Time=38 sec (control=22-44sec), Fasting Blood Sugar=89mg/dl, liver function tests and other findings were not remarkable. Oral aspirin 40 mg per day was started the day before surgery and continued with pyridoxine and folic acid in the morning of operation. The patient was allowed to take water till two hours before surgery in order to avoid preoperative dehydration and intravenous infusion of 5% dextrose in saline was started at 70ml per hour at that time to prevent hypoglycemia and dehydration. Monitoring included precordial stethoscope, electrocardiography, pulse oximetry, end tidal CO2 and noninvasive blood pressure measurement. Serum glucose levels were monitored every two hours. Intravenous fentanyl 1µg/kg was given five minutes before the induction of anaesthesia which was performed with propofol 2.5 mg/kg IV. Tracheal intubation was facilitated with injection of atracurium 0.5mg/kg IV. At this time intravenous lidocaine 1mg/kg and dexamethasone 0.1 mg/kg were injected to attenuate the stress of laryngoscopy and tracheal intubation and the risk of postoperative nausea and vomiting respectively. The trachea was intubated with a cuffed tracheal tube size 5.5 mm. Two lungs were mechanically ventilated with a mixture of air and oxygen (FIO2=50%) and we did not use nitrous oxide. Anaesthesia was maintained with propofol infusion 100 µg /kg/min and intermittent doses of fentanyl and atracurium were injected for more analgesia and muscle relaxation, if needed. At the end of the surgery which lasted one hour, neostigmine 40 µg /kg and atropine 20 µg /kg were given to reverse the residual effects of muscle relaxants and the patient was extubated and transported to the recovery room. We also used automated pneumatic compression stockings and set it to inflate every 15 minutes to promote the venous return of the lower limb prior to the start of operation and continued it for 24 hours postoperatively. Serum glucose levels and coagulation test results were monitored in the recovery room and the next days before discharge. The child had an uneventful postoperative course and was discharged with good condition 48 hours later.
Discussion
Defective function of the enzyme cysthationine synthase results in elevated level of methionine and homocysteine. Elevated level of homocysteine interferes with the normal cross linkage of collagen, which plays an important role in multisystemic manifestations including: ocular (ectopia lentis), skeletal (osteoprosis, methaphyseal changes in the long bones, knock knees, flat feet and kyphoscoliosis) and vascular (thromboembolic diathesis) manifestations [4,5].
The diagnosis of type І homocystinuria is made by confirmation of elevated plasma methionine and homocysteine [2] and by demonstrating homocysteine in the urine as evidenced by the development of characteristic magenta red color upon exposure to nitroprusside [2, 6]. Treatment is aimed at reducing the plasma homocysteine levels by administration of diet with low methionine content and high levels of pyridoxine.
There are three major issues of concern for anesthesiologists: the first one is the increased risk of thromboembolic events. Measures to prevent perioperative thromboembolism include: administration of pyridoxine and dietary measures to lower or control serum methionine and homocysteine levels, [1-7] adequate preoperative hydration, [3-7] reduction in blood viscosity and platelet adhesiveness, maintenance of high cardiac output, [3,4] using pneumatic stockings to prevent venous stasis of blood and establishing good venus return [4], and early postoperative ambulation [3, 5-7]. Pharmacologic measures are: administration of dipyridamole, low molecular weight heparin [1, 3] and aspirin [3-8].
The second important issue is the use of nitrous oxide (N2O). Nitrous oxide irreversibly binds to cobalt in vitamin B12, which is vital to the remethylation of homocysteine to methionine [9]. Administration of N2O prevents methionine synthesis from homocysteine, results in extreme methionine deficiency in the brain and further functional disturbances of the central nervous system [1, 3]. Fatal outcome with N2O exposure in a three-month-old infant with type III homocystinuria has been reported, too [9]. Accordingly, it would seem reasonable to avoid nitrous oxide in children with specific defects of amino acid metabolism like homocystinuria and we did not use N2O in the present patient.
Hypoglycemia, the third anaesthetic problem, is considered to be the result of increased insulin release caused by high levels of methionine, which usually is seen in type Ι homocystinuria [1, 3, 10]. To decrease the incidence of hypoglycemia, blood glucose levels should be monitored frequently, exogenous glucose should be administered during fasting and the fasting period should be shortened [3, 5]. We monitored the perioperative blood glucose level carefully in this patient and administered dextrose containing solutions.
In conclusion, we provided successful anaesthetic management for a patient with classic homocystinuria, during which, we were careful to take measurements against perioperative thromboembolism and hypoglycemia and we also avoided the use of nitrous oxide.
Authors' Contribution
HZ carried out the experiments and interpreted the results, conceived the study, participated in design and edited the final manuscript.
MS carried out the literature search and prepared the draft manuscript, conceived the study, participated in design and edited the final manuscript.
KS designed the study and performed the analysis, conceived the study, participated in design and edited the final manuscript.
MN conceived the study, participated in design and edited the final manuscript.
Conflict of Interests
The authors declare that there are no conflicts of interest
Ethical Considerations
Written informed consent was obtained from the patient for publication of this case report
Funding
None
References
[1]. Yamada T, Hamada H, Mochizuki S, et al. General anesthesia for patient with type 3 homocystinuria. J Clin Anesth 2005; 17:565-7. [Pubmed].
[2]. Rezvani I, Rosenblatt DS. Defects in metabolism of amino acids. In: Kliegman RM, Behrman RE, eds. Nelson textbook of pediatrics. 18th ed. Philadelphia: W.B. Saunders co, 2007: 536-9.
[3]. Saxena KN, Kapoor S, Chopra N, Dua CK. Anesthetic management of a case of homocystinuria. Indian J Anesth 2006; 50: 476-8.
[4]. Lowe S, Johnson DA, Tobias JD. Anesthetic implications of the child with homocystinuria. J clin Anesth 1994; 6:142-4. [Pubmed].
[5]. Deva C, Gombar S, Kapoor A, Dey N. Anesthetic management of a case of homocystinuria. In: The Indian Anesthetists' Forum. [Online ISSN 0973-0311]. Available at: www.theiaforum.org. Accessed April 1, 2005.
[6]. Tantawy H. Nutritional diseases and inborn errors of metabolism. In: Hines RL, Marshcall KE. Stoelting's anesthesia and co-existing disease. 5th ed. Philadelphia: Churchill Livingstone; 2008: 320.
[7]. Teng YH, Sung CS, Liao WW, et al. General Anesthesia for patient with homocystinuria: a case report. Acta Anesthesiol Sin 2002; 40: 153-6. [Pubmed].
[8]. Parris WC, Quimby CW Jr. Anesthetic considerations for the patient with homocystinuria. Anesth Analg 1982; 61: 708-10. [Pubmed].
[9]. Schmitt EL, Baum VC. Nitrous oxide in pediatric anesthesia: friend or foe? Curr Opin Anesthesiol 2008; 21:356-9. [Pubmed].
[10]. Brown BR Jr, Walson PD, Taussig LM. Congenital metabolic diseases of pediatric patients: anesthetic implications. Anesthesiology 1975; 43:197-209. [Pubmed].

