Case Report

A Case of Moderate Burn Associated with Toxic Shock Syndrome and Followed by Hemophagocytic Lymphohistiocytosis
*Ikuo Ota, *Yasuhide Kitazawa, * Takahiro Ueda, *Tomohide Matsushima.
- *Critical Care Medical Center, Kindai University Hospital, Osaka-Sayama, Japan
- Submitted Monday,March 5,2018
- Accepted Thursday,March 29, 2018
- Published Monday,April 2,2018
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
A 60 year-old man was injured by flame burn on his hands and legs, after explosion of the lacquer- can which he handled at his work place. The dermal wound affected 10% of his body surface area. On day 3 of injury, he developed diarrhea and high fever, and fell into septic shock. The patient was treated for shock with fluid resuscitation and administered catecholamine. Renal replacement therapy was also initiated for acute kidney injury. Gram-positive cocci were cultured from his wound exudate and blood samples. He was diagnosed with toxic shock syndrome (TSS), and was administered with Daptomycin, recombinant Thrombomodulin, Antithrombin and intravenous immunoglobulins (IV-IG). These therapies were effective. As pancytopenia appeared on day 11 of injury, we performed bone marrow analysis by aspiration. The bone marrow analysis indicated that the patient had developed Hemophagocytic Lymphohistiocytosis (HLH). We administered IV-IG and Corticosteroid, which were effective.
TSS and HLH have a similar onset mechanism, i.e. the immunological hyper-response to septic stress. However, there are few publications of cases associated with TSS and HLH either simultaneously or sequentially. This clinical report discusses the relationship among burn, TSS and HLH.
Keywords
Toxic shock syndrome (TSS), Hemophagocytosis, Burn wounds, Staphylococcus
aureus
Introduction
Toxic shock syndrome (TSS) is a serious systemic disease that can develop due to TSS-1 or enterotoxin, which is an exotoxin from Staphylococcus aureus and progresses to shock and multiple organ failure in a very short time.
Hemophagocytic lymphohistiocytosis (HLH) covers a wide array of related life-threatening conditions featuring ineffective immunity characterized by an uncontrolled hyperinflammatory response. HLH is often triggered by infection and is caused by hyperactivation of T-cells and macrophage.Because complications of both TSS and HLH are so rare and sometimes life-threatening, prompt diagnosis is needed for success. We report a rare case of TSS associated with HLH after a moderate burn injury.
Case presentation
The patient was a 60-year-old man. He was injured at work-place by flame burn on his hands and legs, when he was handling lacquer-can which suddenly exploded and burning his clothes.
On admission, the patient was alert with stable vital signs. Burned injury was found on both hands and both legs. The burned wounds were assessed as superficial dermal burn (SDB) and deep dermal burn (DDB). The wounds were evaluated as Body surface area (BSA) 10% and burn index 5.0 (Fig 1).
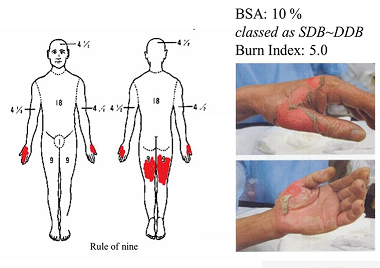

Fig. 1: Burn wound on admission
After hospitalization, the patient was not treated with any antibiotics. The wound was treated with white Vaseline. On day 3 of injury, he developed diarrhea and high fever and fell into septic shock (BP 55/40 mmHg, pulse 95/min). Antibiotics and intravenous immunoglobulin were administered. Moreover, PMX-DHP was performed to remove endotoxin. Renal replacement therapy was initiated for acute renal failure and was continued until day 37 of injury.
On day 6 of injury, skin rash appeared around the trunk. On the same day, the laboratory report showed the presence of Gram-positive cocci in blood and wound exudate from samples taken on day 3 of injury. Therefore, Daptomycin (DAP) 350 mg/day was administered suspecting toxic shock syndrome (TSS) from Methicillin-resistant staphylococci (MRSA). As a result, the infection parameters including procalcitonin (PCT) improved. Desquamation appeared on day 12 of injury and this case met the diagnostic criteria of TSS (Table 1) [1]. Later, MRSA was detected in blood and wound culture.
Table 1: Diagnostic criteria of TSS
| For staphylococcal toxic shock syndrome, the diagnosis is based
strictly upon CDC criteria defined in 2011, as follows |
| 1. Body temperature > 38.9 oC |
| 2. Systolic blood pressure < 90 mmHg |
| 3. Diffuse macular erythroderma |
| 4. Desquamation (especially of the palms and soles) 1–2 weeks after
onset |
| 5. Involvement of three or more organ systems: |
| • Gastrointestinal (vomiting, diarrhea) |
| • Muscular: severe myalgia or creatine phosphokinase level at least
twice the upper limit of normal for laboratory |
| • Mucous membrane hyperemia (vaginal, oral, conjunctival) |
| Kidney failure (serum creatinine > 2 times normal) |
| Liver inflammation (bilirubin, AST, or ALT > 2 times normal) |
| Low platelet count (platelet count < 100,000 / mm3) |
| Central nervous system involvement (confusion without any focal
neurological findings) |
| 6. Negative results of: |
| • Blood, throat, and CSF cultures for other bacteria (besides S.
aureus) |
| • Negative serology for Rickettsia infection, leptospirosis, and
measles Cases are classified as confirmed or probable based on: |
| - Confirmed: All six of the criteria above are met (unless the
patient dies before desquamation can occur) |
| - Probable: Five of the six criteria above are met. |
Despite the improvement of inflammation, thrombocytopenia occurred from day 10 of injury, followed by progressive leukopenia and anemia. Pancytopenia was found on day 12 and performed bone marrow aspiration study.
The bone marrow investigation revealed that the count of nucleated cells was remarkably reduced to 0.2×104, while megakaryocytes were absent. Thirty eight percent of the nucleated cells were macrophages, all of which were phagocytic (Fig 2 ,Table 2). Considering the CBC and laboratory data (soluble interleukin-2 receptor; s-IL2R 6,252 u/mL, triglyceride 296 mg/dL, Fibrinogen 418 mg/dL, Ferritin 1,346 mg/dL), it was diagnosed as HLH (Table 3) [2]. On day 13 of injury, methylprednisolone sodium succinate (MPSS) 1 g/day for three days as a pulse therapy was administered. Thereafter, G- CSF and immunoglobulin were administered (Fig 3). In the bone marrow examination on day 21 of injury, nucleated cell 3.6×104 /mm3, macrophage 0.7% and megakaryocytes 4.0 /mm3 were detected (Fig 4). After the amelioration of HLH, debridement and skin grafting on the burn wounds were performed. The patient was discharged from hospital 88 days after admission.
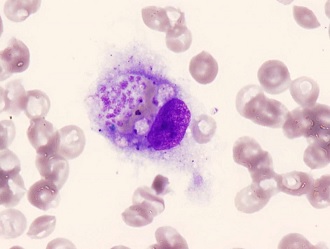

Fig. 2: Bone marrow on day 12 of injure showing a macrophage with hemophagocytosis
Table 2. Finding of bone marrow aspiration
.auto-style1 {
text-align: center;
}
| Blood data |
| Hb |
8.3 g/dL |
| Platlet |
1.1 x 104 /mm3 |
| WBC |
2.4 x 103 /mm3 |
| TG |
296 mg/dL |
| Fibrinogen |
418 mg/dL |
| sIL2R |
6,252 IU/mL |
| Ferritin |
1,346 ng/mL |
| Bone marrow aspiration data |
| NCC |
0.2 x 104 /mm3 |
| %Macrophage |
38 % |
| %Phagocytosis |
100 % |
| Megakaryocyte |
0 /mm3 |
Table 3: Diagnostic criteria of HLH (HLH 2004).
| (1) A molecular diagnosis consistent with HLH |
| (2) "Diagnostic criteria for HLH fulfilled (five out of the eight
criteria below)" |
(A) "Initial diagnostic criteria (to be evaluated in all patients
with HLH)"
Fever
Splenomegaly Cytopenias (affecting > 2 of 3
lineages in the peripheral blood): Hemoglobin < 90 g/L (in infants <4
weeks: hemoglobin <100 g/L) Platelets < 100 x 109/L Neutrophils < 1.0 x
109/L
Hypertriglyceridemia and/or hypofibrinogenemia: Fasting
triglycerides 3.0 mmol/L (i.e., > 265 mg/dL) Fibrinogen < 1.5 g/L
Hemophagocytosis in bone marrow or spleen or lymph nodes
No evidence
of malignancy
|
(B) New diagnostic criteria
"Low or absent NK-cell activity
(according to local laboratory reference)"
Ferritin > 500 mg/L
Soluble CD25 (i.e., soluble IL-2 receptor) > 2,400 U/mL
|

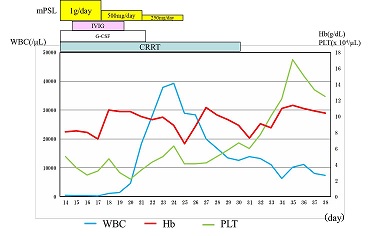
Fig. 3: Changes in Blood Cell Count after start of HLH treatmentIt took 4, 9 and 20 days to recover anemia, leukocytopenia and thrombocytopenia respectively.

Fig. 4: Bone marrow viewed on day 21NCC: 3.6×104 /μL, Macrophage: 0.7 %, Megakaryocyte: 4.0 /μL
Discussion
Toxic shock syndrome (TSS) was first described in 1978 by Todd et al. [2]. Toxic shock syndrome toxin-1 (TSST-1) and the streptococcal enterotoxin are classed as super antigens. These are proteins that can over-activate the immune system by bypassing the usual steps in the antigen-mediated immune response sequence. Through this mechanism, they can cause massive T-cell stimulation and an overwhelming immune cascade that is destructive to all end organs. The normal route by which T- cells are activated by antigens is to process a molecule called the major histocompatibility complex (MHC).
The MHC then presents the processed antigen to the inner groove of the T-cell [4, 5]. In contrast, super-antigens can bind to the outer groove of the MHC without this process and can bind to the beta chain on the T-cell receptor. The result is the production of huge quantities of cytokines such as tumor necrosis factor, interleukins and gamma interferon which are responsible for the overwhelming cell destruction and systemic disorder as seen in TSS [4, 6]. Staphylococcal TSS can occur with any site of Staphylococcal infection. In our diagnosis, TSS may have been caused by burn wound sepsis. Also, early diagnosis of TSS is difficult because characteristic desquamation of the skin following erythema appears a week or more later.
Detection of causative toxins is useful for definitive diagnosis. We were able to confirm the results of positive TSST-1, positive type III coagulase and positive C-type enterotoxin in the blood sample on the day of onset. The risk of TSS is extremely rare for persons over 50 years old, because most of them would have acquired TSST-1 antibodies [7]. However, there are few reports of TSS in adult patients with burns [8]. Among the antibacterial agents, anti-MRSA drugs are recommended for TSS [9]. Moreover, the human immunoglobulin has a TSST-1 neutralizing effect. DAP was chosen as anti-MRSA antibiotics, and immunoglobulin was added in this case.
The endotoxin absorption therapy using polymyxin B–immobilized fiber direct hemoperfusion (PMX-DHP) is known to be effective against septic shock not only with Gram-negative bacterial infection but also with Gram-positive bacterial infection. It is considered to remove the mediators such as Anandamide and High Morbility Group Box 1 (HMGB-1) [10]. Although PMX-DHP was performed early on this case, any hemodynamic improvements were not obtained. We found normal level of serum endotoxin even during shock state later.
Scott et al. [11] reported a case of patient suffering from hemophagocytic lymphohistiocytosis (HLH) in 1939. Since then Risdall et al. [12] have reported that some viruses may cause HLH in1979, various studies have been published to date.
HLH can be caused by various infections and characterized by pancytopenia and histio-monocytic proliferation and phagocytosis in the bone marrow and the lymphoid system [13, 14]. It has been suggested that the pathogenesis of this syndrome is uncontrolled T-cell activation, which causes over secretion of Th1 cytokines such as IFN-γ and IL-2, further activating T cells and monocytes/macrophages [15]. Clinical conditions including pancytopenia, coagulopathy and organ failure are considered to result from hyper-cytokinemia [16]. HLH is classified into primary/hereditary and secondary/reactive types.
Secondary HLH has a variety of causes including viral infection, bacterial infection, malignancy, autoimmune diseases and drug-induced disorders [17]. Our case was diagnosed as infection-induced type HLH. It is reported that survival of 60 to 70% is expected in infection-related HLH except for the EBV-related type [18], which often falls into a critical state [19]. Therefore, strong immuno- chemotherapy should be chosen for critical cases. HLH-2004 chemo-immunotherapy includes etoposide, cyclosporine A [2]. Rituximab can also be another therapeutic option. However, they are not covered by insurance allowed for HLH in Japan. It is usual to administer steroid and IVIG for bacteria-related HLH.
Observing that thrombocytopenia was prolonged after the recovery from TSS in this case, we initially suspected sepsis induced DIC. Subsequently, when pancytopenia appeared, we suspected HLH. To control the hyper-cytokinemia associated with HLH, immuno-modulation therapy including steroid pulse therapy were administered. Based on HLH guideline 2004, this case was finally diagnosed as HLH.
An over-activation of T-cells followed by the cytokine storm plays a major role in both TSS and HLH. It is unclear why there are so few clinical reports on TSS cases associated with HLH either simultaneously or sequentially.
Conclusion
This clinical report shows that TSS case from moderate burns, after being treated, can progress to HLH. Because complications of TSS and HLH are both so rare and sometimes life-threatening, this case teaches that proper clinical reasoning and prompt diagnosis can bring success.
Acknowledgements
This report had already been published on Japanese Journal of Burn Injuries (JJBI); Ikuo Ota et al, JJBI 2016; 42: 19-25 (written in Japanese). This manuscript in English was submitted under the permission of Japanese Society for Burn Injuries (JSBI).
Reference
[1].Davis JP, Chesney PJ, Wand PJ et al: Toxic shock syndrome: epidemiological features, recurrence risk factors and prevention. New Engl J Med 1980; 14: 1429-1435.[PubMed]
[2].Henter JI, Horne AC, Arico M et al: Hemophagocytic lymphohistiocytosis. Pediatric Blood Cancer 2007; 48: 124-131.[PubMed]
[3]Todd JK, Fishalt M, Kapral F et al: Toxic shock syndrome associated with phage group 1 staphylococci. Lancet 1978; 312: 1116–1118.[PubMed]
[4]Issa NC, Thompson RL: Staphylococcal toxic shock syndrome. Postgrad Med 2001; 110: 55-62.[PubMed]
[5].Edwards-Jones V, Shawcross SG: Toxic shock syndrome in the burned patient. Br J Biomed Sci 1997; 54: 110-117.[PubMed]
[6].Andrews MM, Parent EM, Barry M et al: Recurrent non-menstrual toxic shock syndrome: clinical manifestations, diagnosis and treatment. Clin Infect Dis 2001; 32: 1471-1479.[PubMed]
[7].Vergeront JM, Stolz SJ, Crass BA et al: Prevalence of serum antibody to Staphylococcal enterotoxin F among Wisconsin residents implication for toxic shock syndrome. J Infect Dis 1983;148:692-698.[PubMed]
[8].Withey SJ, Carver N, Frame JD et al: Toxic shock syndrome in adult burns. Burns 1999; 25: 659-662.[PubMed]
[9].Silversides JA, Lappin E and Ferguson AJ: Staphylococcal Toxic Shock Syndrome: Mechanisms and Management. Curr Infect Dis Rep 2010; 12: 392–400.[PubMed]
[10.Nakamura T, Sato E, Fujiwara N, et al: Suppression of high-mobility group box-1 and receptor for advanced glycation end-product axis by polymyxin B–immobilized fiber hemoperfusion in septic shock patients. Journal of Critical Care 2011; 26, 546–549.[PubMed]
[11].Scott RB, Robb-Smith AHT: Histiocytic medullary reticulosis. Lancet 1939; 234: 194-198. https://doi.org/10.1016/S0140-6736(00)61951-7
[12].Risdall RJ, Mckenna RW, Nesbit ME et al: Virus-associated hemophagocytic syndrome: a benign histiocytic proliferation distinct from malignant histiocytosis. Cancer 1979; 44: 993-1002.[PubMed]
[13].Sullivan JL, Woda BA. Lymphohistiocytic disorders. In Nathan DG. Oski FA, eds. Hematology of infancy and childhood, 4th ed. Philadelphia, PA, Saunders, 1993, 1354-1374.
[14].Cline MJ: Histiocytes and histiocytosis. Blood 1994; 84: 2840-2853.[PubMed[ [Free Full Text]
[15].Oyama Y, Amano T, Hirakawa S et al: Haemophagocytic syndrome treated with cyclosporine A: T- cell disorder Br J Haematol 1989; 73: 276-278.[PubMed]
[16].George MR: Hemophagocytic lymphohistiocytosis: review of etiologies and management. Journal of Blood Medicine 2014; 5: 69–86.[PubMed] [PMC]
[17].Larroche C: Hemophagocytic lymphohistiocytosis in adults: diagnosis and treatment. Joint Bone Spine 2012; 79: 356-361.[PubMed]
[18].Reiner AP, Spivak JL: Hemophagocytic histiocytosis: A report of 23 new patients and a review of the literature. Medicine 1988; 67: 369-388.[PubMed]
[19].Kitazawa Y, Saito F, Nomura S et al: A case of Hemophagocytic Lymphohistiocytosis after the primary Epstein-Barr Virus infection. Clinical and Applied Thrombosis/Hemostasis 2007; 13: 323-328.[PubMed]

