Review

Role of imaging in Female Infertility
1 Pushpa Ranjan, 2 Rajeev Ranjan
- 1Department of Radiology, IGIMS, Patna, India
- 2Department of Surgery, NMCH, Sasaram, India
- Submitted: 5 june 2016
- Accepted: 2 october 2016
- Published: 23 December 2016
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Imaging plays key role in diagnostic evaluation of female infertility. Infertility has many causes – tubal and peritubal disorders, uterine disorders including mullerian duct anomalies, ovarian disorders, cervical disorders. There is increase in incidence of female infertility in association with that there is increase in demand for female imaging modalities. Although HSG is gold standard investigation but USG is first line investigation for female infertility.Sonosalpingography differentiate among uterine synechiae, endometrial polyps and submucosal leiomyomas. Pelvic MRI is very sensitive for detection of endometriosis. It is also used for detection of adenomyosis, leiomyomas, mullerian duct anomalies. Cervical stenosis is referred as failure or difficulty in cervical canulation in HSG. Ovarian disorders are usually diagnosed by USG. Multimodality imaging approach with appropriate selection of imaging modality is necessary to know exact cause of infertility because it direct subsequent management of infertility.
Sonosalpingography differentiate among uterine synechiae, endometrial polyps and submucosal leiomyomas. Pelvic MRI is very sensitive for detection of endometriosis. It is also used for detection of adenomyosis, leiomyomas, mullerian duct anomalies. Cervical stenosis is referred as failure or difficulty in cervical canulation in HSG. Ovarian disorders are usually diagnosed by USG. Multimodality imaging approach with appropriate selection of imaging modality is necessary to know exact cause of infertility because it direct subsequent management of infertility.
Key words:
infertility, hysterosalpingography, ultrasonography, magnetic rasonance imaging
Introduction
Infertility is defined as failure to conceive a desired pregnancy after 12 months of unprotected intercourse. It is of two types – primary and secondary infertility. Primary infertility in which a couple never have a child. Secondary infertility is failure to achieve a child following a previous pregnancy. Approximately 10% of couple are infertile. Male and female are equally affected. Potential causes of infertility are numerous and they can be categorized as –
Tubal causes – Infection, obstruction
Uterine Causes – Congenital anomalies, infections, uterine synechiae, focal lesions, intrauterine scar.
Ovarian causes – Follicular and ovulation abnormalities includes pituitary adenoma, polycystic ovarian syndrome, premature ovarian failure, gonadal dysgenesis and endometriosis.
Cervical causes - Cervical stenosis caused by Infection, trauma.
Imaging modalities of infertility
Ultrasonography (USG) (mainly transvaginal) It is first line investigation for infertility and can be coupled with colour Doppler, 3D, 4D. It is easily available, low cost and radiation free. Limitations are subjective variations, limited field of view, obesity, gaseous abdomen, small ovaries, fallopian tube and broad ligament are not properly visualised.USG plays important role in interventional procedure. It guides oocyst retrieval, embryo transfer in in-vitro fertilization procedure, drainage of pelvic collection, cyst and abscess.
Sonosalpingography (sono – USG):- It is airless, sterile, saline infusion through soft plastic catheter into cervix with simultaneous endovaginal US. It confirms tubal patency and allows excellent visualization of endometrial cavity and its lining. It differentiate among endometrial polyps, submucosal leiomyomas and intrauterine adhesions.
Hysterosalpingography:-
It is radiological procedure used to demonstrate uterine and fallopian tube lumen using contrast medium. It is valuable technique in evaluation of female infertility because it provides tubal patency. It involves ionising radiation. Despite development of other diagnostic modalities such as MRI, hysteroscopy, laparoscopy, it remains main examination for fallopian tube patency in developing countries [3]. Uterine filling defect and contour abnormalities may be discovered at HSG but typically require further characterization with pelvic USG, pelvic MRI.
Selective Salpingography and fallopian tube recanalzation:- Tubal blockage is diagnosed and recanalized non-surgically. This procedure doesn’t require incision. Fallopian tube is directly opacified by injecting contrast media through catheter placed in tubal ostium under fluoroscope guidance. If fallopian tube obstruction found, a 3-F catheter over 0.015-inch guide wire is directly advanced through obstruction via cornual catheter and obstruction is recanalized (16).
MRI:- It provides excellent soft tissue characterisation of pelvic structure. It detect lesions like adenomyosis, endometriosis, leiomyomas, tubal disease, mullarian duct anomalies, ovarian pathology.[3]It also detect extra-pelvic causes involved in infertility like pituitary adenoma. MRI predicts the outcome of conservative treatment of adenomyosis, leiomyomas and endometriosis and help in better selection of treatment plan.
Recenty, MRI based HSG has been introduced and is superior to USG for studying tube. MRI has potential to replace HSG and laparoscopy in diagnosis of abnormalities of female genital tract [3]. Due to limited availability, and high cost MRI based HSG not become popular.
Sub-centimetric uterine lesions are not detected in MRI. It is contraindicated in patient with pacemaker, cochlear implant.
Causes of infertility
Fallopian Tube Abnormalities:- It is most common cause of female infertility, accounting for 30 – 40 % of cases [1]. Hysterosalpingography, provides optimal depiction of FT, allowing detection of tubal patency, occlusion, irregularity and peritubal disease.
Appropriate steps in imaging evaluation of fallopian tube abnormalities are given below:-
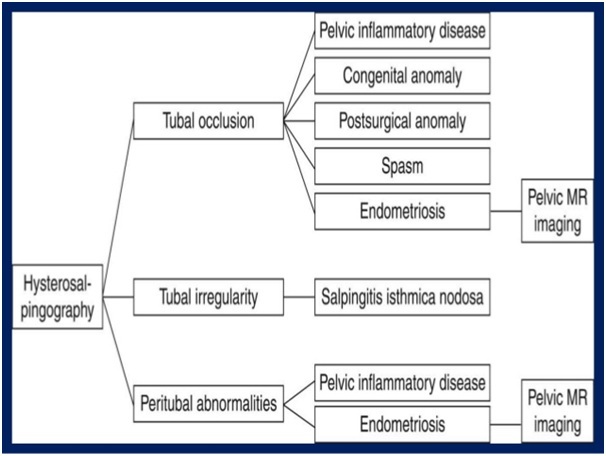
Occlusion of FT occurs at any site of long course of tube. Differential diagnosis of tubal occlusion includes tubal spasm, infection, prior surgery. When tubal occlusion is in the proximal or interstitial part of FT at HSG, tubal spasm should be suspected [1].
When occlusion occurs at ampulla of FT, hydrosalpinx seen, a condition most commonly caused by pelvic inflammatory disease. At hysterosalpingography, tube appears dilated, tortuous, fluid filled, with absence of intraperitoneal spillage of contrast (Fig 1)
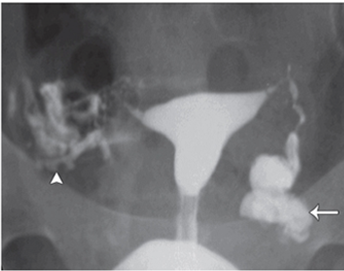
fig 1 left hydrosalpinx HSG show dilated left fallopian tube with abscence of intrapentoneal sppillage of contrast material.right fallopian is patent with free sppillage of contrast material
Pelvic inflammatory disease may lead to peritubal adhesion, is one of the common causeof infertility and manifest as pelvic collection and tubo-ovarian mass. (Fig 2).

Fig 2 right peritubal adhesion deu to previous pelvic infllamtary infection (a) early (b) late HSG show normal filling of contrast in right fallopian tub and a rounded callection of leaked contrast matterial abjaecent to ampullary portion of right fallopian tub the collection is due to peritubal adhesion left fallopian tube is normal and patent
Uterine Adhesion:- Infertility secondary to uterine adhesion is known as Asherman syndrome. Uterine adhesion must be differentiated from normal uterine fold which also appear as longitudinal filling defect when uterine cavity is not fully distended. Sonosalpingography has greater sensitivity than HSG in diagnosing uterine adhesion which show echogenic bands that traverse the endometrial cavity.
Uterine Cause :- Endometrial polyp Even sub-centimetric endometrial polyp and submucosal leiomyoma contribute to infertility by interferring with embryo transfer and implantation. Sonosalpingogrphy show endometrial lesions that are not visible with pelvic ultrasound and it differentiate endometrial polyp from submucosal leiomyoma (Fig 3).

Fig 3uterine polyp (a) spot radiograph in early filling phase show well defined intrautrine filling defect (b) shonosalpingography show posterior endometrial fundad polyp.(c) TVS with doppler show central feeding vessel.
Endometrial polyp typically appear as echogenic intracavitary mass. It allow accurate assessment of its number, location and provides subsequent guidance for hysteroscopic biopsy and excision [1].
Submucosal leiomyomas appear as hypoechoic masses that distort normal endometrium .
Adenomyosis:- is not a common cause of infertility. The frequency of symptomatic adenomyosis peaks between 33 – 50 years, is most often found in parous women. Nulliparous women sometimes affected and experience infertility. Exact cause of infertility in patient with adenomyosis is not known. Adenomyosis may be associated with infertility due to impaired uterine contractility which is necessary for directed sperm transport through uterus (6). It reduced uterine and endometrial reception in enlarged uterus [1]. Both TVS and MRI allow accurate, non-invasive diagnosis of adenomyosis. The relevant diagnostic US findings are :-
(a)The thickening and asymmetry of anterior and posterior uterine mass.
(b)A poorly defined area of decreased or increased echogenicity, heterogenous echotexture, or myometrial cyst [8].
MR imaging criteria include:-
(a)Myometrial wall of low signal intensity with indistinct margins on both T1 and T2W images.
(b)Diffuse or focal widening of junctional zone thickness on T2W images. A junctional zone thickness of 12mm or more optimize the accuracy of MR imaging for this diagnosis.
(c)Punctuate high signal intensity, which corresponds to ectopic endometrium, are often demonstrated on T2W images [4], (Fig 4).

Fig 4 adenomyosls.saghittal T2-weighted MR image show focl of highscgnal intensity indicate ectopic endonetrial gland with widening of junctional zone
Leiomyomas (Fibroids):- Infertility may results when leiomyomas are numerous or have submucosal location that interferes with embryo transfer and implantation. Patient with multiple leiomyoma are at increased risk for early spontaneous fetal loss. If leiomyoma located near uterine cornua, it obstructs ipsilateral fallopian tube and causes absence of fallopian tube opacification[1].
On USG leiomyomas have variable appearance. Uterus may be enlarged. Localised leiomyoma appears as well defined, hypoechoic (Fig 5a)or heterogenous in echotexture which may have hyperechoic calcification. Leiomyomas may demonstrate acoustic attenuation or shadowing without a descrete mass making it impossible to estimate size [15]. Colour Doppler reveals peripheral vascularity of mild to moderate resistance, differentiate it from adenomyoma which reveal moderate central and peripheral vascularity [2].
Transvaginal US is as effective as MRI for detection but MR imaging outperforms transvaginal US in preoperative evaluation of location, numbers and size of fibroid. Sonohysterography demonstrate relationship between endometrium and submucosal fibroid and serve as important adjunct to TVS. On MRI it appears as sharply marginated mass that typically has lower signal intensity than myometrium on T2W(Fig 5b). MRI is highly accurate modality for differentiation of leiomyoma from adenomyosis in case of enlarged uterus; accuracy is 99% [4].

Fig 5 submucosal fibroid spot radiogroph show (a) well defined intraut erine filling defect at fun dus in early filling stag of HSG (b) less apperent fibroid van uterus is more distance (c0 large fibroid disorating uterine cavity .(d)sonosalpingogrmn show salin out line the submucosal fibroid
Mullerian Duct Anomalies:- If other causes of infertility are excluded, uterine anomalies may be suggested as cause of infertility. Mutliplanar MRI is diagnostic. Millerian Duct Anomalies are classified according to American Society of Reproductive Medicine System [2], Fig 6]
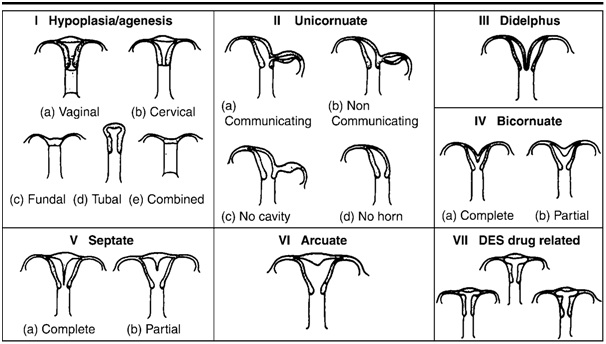
Fig 6 hysterosalpinography showing vevarious anumolies seen in the uterus and fallopian tube
Classification of mullerian duct anomalies developed by American System of Reproductive Medicine
The anomaly can be detected because of amonorrhoea, before HSG performed.
Class II: - Unicornuate uterus:
That represents partial or complete hyperplasia of one of mullerian duct. In rare cases of degeneration of the masonephric duct, the uterine cavity appears monocular when imaged, placed right or left of midline. The unicornuate uterus contacts only the coordinate fallopian tube.
Class III :- Diadelphys uterus:
This is rare abnormality that results from complete nonfusion of mullarian duct and includes the duplication of uterine cavity, cervix and vagina. Rarely this uterus has a single vagina.
Class IV: - Bicornuate uterus:
It demonstrates incomplete fusion of superior segment of uterovaginal canal. The uterine cavity is divided into two, each half has a narrow length, shape and stand apart from each other. MRI confirm the presence of bicornuate uterus by showing intercornual distance of > 4 cm and concavity of fundal contour or an external fundal cleft >1 cm..
Class V:- Septate uterus:. It represent partial or complete failure of resorption of the uterovaginal septum after failure of mullarian duct. MR imaging help to differentiate a septate uterus from bicornuate uterus by depicting a normal convex, flat or minimally concave (< 1 cm deep) external fundal contour with septate uterus. MR imaging readily demonstrate composition and extent of septum.
Class VI: Arcuate uterus:
Near complete resorption of uterovaginal septum resulting in shallow, smooth, broad based impression on uterine cavity which may be depicted in HSG, US and MRI.
Class VII or DES (diethyibestrol) induced– Exposure to this synthetic estrogen
antenatally can result in a T -shaped, hypoplastic, and constricted uterus.
Endometrioma:- It is found in 25–50% of infertile women and 30-35%of women with endometriosis are infertile [4].The condition almost exclusively affects women during reproductive age. This condition mostly involve ovary but secondarily involve other pelvic organs[10]. Laproscopy is mainstay for diagnosis, staging and treatment of endometriosis. Imaging modality for endometriosis include Pelvic US, MRI. Endometriosis may take in the form of small implant or cyst that change in size and appearance during menstrual cycle and may initiate inflammatory response leading to fibrosis and adhesion [12]. Endometriotic cyst referred as endometrioma, result from repeated haemorrhage within implant. USG has low sensitivity for detection of focal implants. Endometrioma located in ovary show well defined unilocular or multilocular, predominantly cystic lesion containing homogeneous low level internal echoes. It may appear fluid-fluid level. Small linear echogenic foci may be present in the wall of cyst [15] [Fig 7)].
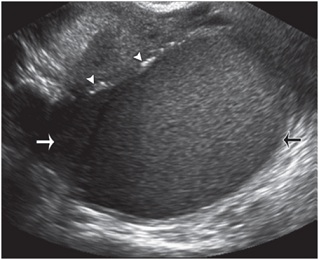

Fig. 7 Endometrioma. Sagittal transvaginal USimage obtained in a woman with a history of endometriosis shows an ovarian mass with multiple fine internal echoes (arrows) and several hyperechoic mural foci (arrowheads).
MRI is more sensitive for detection of endometrioma which show (a) adnexal cyst of high signal intensity on both T1W and T2W image. (b) High signal intensity on T1w image and low signal intensity on T2w image. Dense concentration of cyclic haemorrhage and high viscosity of contents in endometrioma causes T2 shortening and produces shadowing. Fat suppressed T2W image are very useful for detecting peritoneal implants. Tube may be involve in the form of hydrosalpinx or with peritubal adhesion while uterine involvement as adenomyosis. Adhesion is seen on MRI as hypointense strands within adjacent fat, obscuring adjacent interface. A posteriorly displaced uterus, kissing ovaries, elevated posterior fornix, angulated small bowel loop,hhydro/haematosalpinx and multilocular fluid collections are indirect indicator of pelvic adhesion [2].
Ovarian Abnormalities:- disorder affecting ovulation account for 30 to 40% of cases of female infertility [4]. Ovarian causes of infertility include primary ovarian cause and secondary ovarian cause. Primary ovarian cause includes nonfunctional ovaries, premature ovarian failure and absense of ovaries (gonadal dysgenesis). These conditions are usually diagnosed on the basis of clinical and biochemical findings. Secondary ovarian cause includes polycyctic ovarian disease, endometrioma and ovarian cancer. Imaging is more valuable for diagnosing secondary ovarian cause. Pelvic USG is usually performed for initial eveluation of ovaries.
Polycystic ovarian Syndrome: - It is one of the most common causes of female infertility, accounting upto 8% of women [14]. Diagnosis of polycystic ovarian syndrome is based on hormonal imbalance and laboratory findings. Patients with this syndrome often demonstrate abnormal ratio of LH abd FSH. Clinical manifestation includes hirsutism, anovulation and infertility [4]. 2003 joint ESHRE/ASRM meeting in Rotterdam consensus criteria required at least two of the following three conditions to be present for diagnosis of PCOS (a) o ligo- or anovulation (b) clinical and biochemical hyperandrogenism (c) polycystic ovaries [13].
USG criteria for polycystic ovaries are present when (a) one or both ovary demonstrate 12 or more follicles measuring 2-8 mm in diameter Fig 8 a)) (b) ovarian volume exceeds 10mm (c) increased echogenicity of the ovarian stroma is the most sensitive and specific sign but the finding is subjective. Only one ovary meeting either of these criteria is sufficient [13]. According to American College of radiology Appropriatness criteria, pelvic MR imaging is generally recomended in case in which TVS and transabdominal US does not offer adequat visualization. In MRI, ovaries in patients with PCOS demonstrate low signal intensity central stroma surrounded by small peripheral cyst hyperintense on T2W. (Fig 8b))

Fig.8 Polycystic ovary syndrome. (a) Transvaginal US of the ovary shows multiple peripheral subcentimetric follicles (arrow). (b) Coronal T2-weighted MR image of same patient shows bilateral ovarian enlargement with multiple peripheral follicles (arrows).
Pituitary Adenoma:- Hyperprolactinemia can be cause of infertility and associated with diminished gonadotropin secretion, secondary amenorrhoea and galacrorrhoea. Patient should be examined first for drug induced hyperprolactinoma before any infertility work up is initiated. For example antidepressant, cimetidine, dopamine antagonist, reserpine, varapamil etc. are known to interfere with prolactin secretion. MRI is modality of choice for depecting pituitary adenoma.(Fig 9(a) (b) ) Microadenoma (<10mm) is usually hypointense to normal pituitary gland on T1W image. Convexity of pituitary contour and deviation of stalk is indirect sign of pituitary adenoma. Dynamic postcontrast MRI show strong enhancement of normal pituitary gland and its stalk in early phase of dynamic imaging whereas relatively weak enhancement seen in microadenoma. Macroadenoma (>1mm) may compress or invade surrounding structure including optic chaisma, cavernous sinus and bony sella[4].
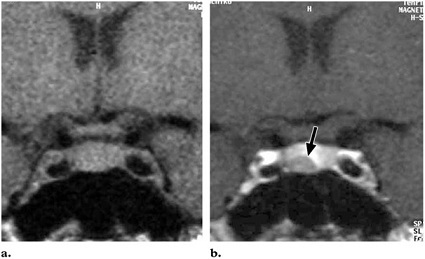
Fig.9 Pituitary microadenoma. (a) Coronal T1-weighted image shows a pituitary gland that is not enlarged.(b) Coronal T1-weighted image obtained after intravenous bolus injection of contrast material shows a mass with decreased enhancement(arrow) in the pituitary gland.
Cervical Abnormalities: - cervical factor infertility imply indequate quality or volume of cervical mucus, a condotion that account for approximately 10% of cases of female infertility. Patient with this condition suggested postcoital test that does’t involves imaging.
Cervical Stenosis: - clinically defined as cervical narrowing that prevents insertion of 2-5mm wide dilator.This condition may be congenital or acquired secondary to infection or trauma.
Consequenses of cervical stenosis include obstruction of menstrual outflow with resulting amenorrhoea, dysmenorrhoea, and infertility due to inability of sperm to enter the upper genital tract. Cervical factor may be serious hindrance to assisted infertility technique including embryo transfer and intrauterine insemination.At HSG cervical stenosis may appear as narrowing of andocervical canal (normal diameter 0.5 to 3.0 cm) or it may manifest as complete obliteration of cervical os, preventing insertion of HSG catheter. Masses such as cervical polyps, fibroids and neoplasm also cause narrowing of cervical canal [1].
Conclusion:
Infertility has multifactorial causes like tubal, uterine, ovarian, and cervical. A systematic approach of diagnosis is pelvic USG followed by HSG, pelvic MRI. . Multimodality approach may be needed to know the cause of infertility. Selection of appropriate imaging modality is essential because it guides subsequent managment. MRI used in case of complex manifestation and when USG findings are normal and equivocal. MRI may be servae as adjunct to diagnostic laproscopy and HSG in patient with tubal abd peritubal diseases. A normal HSG finding in case of infertility suggest extrauterine cause.
References:
[1].Fill A. Steinkeler, MD, Courtney A. Woodfield, MD, Elizabeth lazarus, MD, Marry m. Hillstorm, MD. Female infertility: A systemic Approach to Radiological and Diagnosis. Radiographics 2009; 29 : 1352 – 1370.
[2]Rajul Rastogi. Role of imaging in Female infertility. Indian Journal of radiol imaging. Aug 2010, 20 [3] : 168 – 173.[pubmed] [PMC Full text]
[3].Khaled Abdel Malek, MD, Mohammed Hassan, MD, Ahmed Solimen, MD, Hebal El-sawah MD, Amar Osama Azab, MD. A Prospective Comparative Study to Assess the Accuracy of MRI Versus HSG in Tubouterine causes of Female Infertility. Middle East Fertility Society Journal. Vol. 2, No. 3, 2003.
[4]Izumi Imaoka, MD, Akihiko wada, MD, Michimasa Matsuo, MD, Masumi Yoshida, MD, Hajime Kitagashi, MD, Kazuro Sugimura, MD. MR Imaging of Disorder associated with Female Infertility: Use in Diagnosis, Treatment and management. Radiographics 2003, 23 : 1401 – 1421.[Pubmed
[5]Sabastein Novellas, Madleen Chassang, Jerome delotte, Olivier Toullalan, anne Chevallier, Jerome Bouaziz, Patrick Chevallier. MRI Characteristics of the Uterine Junctional Zone : From Normal to the diagnosis of Adenomyosis. AJR 2011; 196 : 1206 – 1213.[Pubmed]
[6]Ken Tamari, MD, Kaori Togashi, MD, Tsuyoshi Ito,MD, Nobuko Morisawa, MD, Toshitaka Fujiwara, MD, Takashi Koyama, MD. MR Imaging Finding of Adenomyosis : Correlation with Histopathologic Feature and Diagnostic Pitfall. Radiographics 2005; 25 : 21 – 40.[Pubmed]
[7]Caroline Reinhold, MD, Faranak Tajazoli, MD, Amiro Mehio, MD, Lin Wang, MD, Mostafa Atri, MD, Evan s seigelman, MD, Lori Rohoman, ACR, RTMR. Uterine Adenomyosis : Endovaginal US and MRImaging Feature with Histopathologic Correlation. Radiographics 1999; 19 : S1 47 – S 160.[Pubmed]
[8]Caroline Reinhold, Faranak Tajazoli and Lin Wang. Imaging Feature of Adenomyosis. Human Reproduction Update. 1998 vol. -4, No. – 4, pp 337 – 349.?[pubmed]
[9]Athanasios Chalazonitis, MD, PhD,MPH, Ioanna tzovara, MD, Fotios Laspas, MD, MSc, Petros Porfyridid, MD, Nikos Ptohis, MD, PhD, Georqios Tsimitselis, MD. Hysterosalpingography : Technique and Applications. Current Problem in Diagnostic Radiology. Vol. 38, Issue 5, Pg- 199 – 205, Sept – Oct 2009.[Pubmed]
[10]Genevieve L Bennett, Chrystia M Slywotzky, Mariella Cantera and Elizabeth M Hecht. Unusual Manifestation and Complication of Endometrioma – Spectrum of Imaging findings : Pictoral Review. American Journal of Roentgenology. Vol. 194, Issue 6, June 2010.
[11]M. A. Shafeek, MI Osman, M. A. Hussain. The Hysterosalpingographic Diagnosis of Stromal Endometrioma. Clinical Radiology Vol. 29, Issue – 5, Pg- 565 – 569
[12]- Evan s. Siegelman, MD, Edward R. Olivier, MD, PhD. MR Imaging of Endometrioma : Ten Imaging Pearls. Radiographics 2012; 32 : 1675 – 1691.[pubmed]
[13]Tony T Lee, MD, Marry E Rausch, MD, MSCE. Polycystic Ovarins Syndrome : Role of Imaging in Diagnosis. Radiographics 2012 ; 32 : 1643 – 1657. [pubmed]
[14]Jeffery Dee Olpin, Anne kennedy. Secondary infertility in women : radiologic evaluation. Reports in Medical Imaging. 10 Jan 2011: 41 -14.
[15]:- Diagnostic Ultrasound. Carol M. Rumack, Stephanie R. Wilson, J. William Charbonaen. Associate Editor Jo – Ann M. Johnson. Fourth Edition.
[16]Amy S Thurmond, Lindsay S Machan, Antonie J maubon, Jean-Pierre Rouanel, David M Hovsepian, Arl Van Moore, Ronald J Zagoria, Kavin W Dickey, James C Bass. A Review of selective Salpingography and Fallopian Tube Catheterization. Radiographics 2000; 20:1759- 1768.[pubmed]

