Original Article

The Effect Of Lagenaria Siceraria (Molina) On Acute Lung Injury Induced By Oleic Acid In Rats
1Fahri Yetişir, 2A. Ebru Salman, 3İ.Özkan Önal, 4Dilara Zeybek, 2Mustafa Aksoy, 3Ahmet Dostbil, 5Halit Yetişir, 4F. Figen Kaymaz, 6Süheyla Ünver, 7Mehmet Kiliç
- 1Department of General Surgery, Atatürk Research and Trainning Hospital, Ankara
- 2Department of Anesthesiology and Reanimation, Atatürk Research and Trainning Hospital, Ankara
- 2Etlik Research and Trainning Hospital
- 4Department of Histology and Embryology, Hacettepe University Faculty of Medine, Ankara
- 5Department of Horticulture Melikgazi, Erciyes University Faculty of Agriculture, Kayseri
- 6Department of Anesthesiology and Reanimation, Oncology Research and Trainning Hospital, Ankara
- 7Department of General Surgery, Yıldırım Beyazıt University, Ankara
- Submitted: February 13, 2013
- Accepted: March 09, 2013
- Published:April 08, 2013
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Aim
Oleic acid has been used to induce acute lung injury in experimental studies. Lagenaria siceraria (Molina) has been reported to have cardiotonic, hepatoprotective, immunomodulatory, antihyperglycemic, antihyperlipidemic, analgesic and anti-inflammatory properties. In this study, we investigated the effects of Lagenaria siceraria (LS) fruit juice on acute lung injury induced by oleic acid in rats.
Material and Method
Thirty five adult female Sprague Dawley rats divided into 5 groups, 7 in each. Group I and group II received normal saline for 30 days, group III, IV and group V received LS at a dose of 200 mg/kg, 200 mg/kg and 400 mg/kg for 30 days by gavage respectively. 100mg/kg oleic acid was administered i.v in group II, group IV and group V. Histopathological examination of the lung was performed with light and electron microscopy. Levels of protein carbonyl, malondialdehyde, superoxide dismutase, catalase and glutathione peroxidase levels were measured in tissue samples. Levels of TNF alpha, IL10, IL6 and total tissue oxidant status and total tissue antioxidant status were measured in serum samples.
Results
Light microscopy showed that LS at both doses decreased the total lung injury score compared to group II. In electron microscopy, LS at both doses decreased the thickness of the fused basal lamina when compared to group II. TNFα, IL6 in serum and MDA in tissue were higher in group II when compared to group me and attenuated in group V significantly.
Conclusion
Lagenaria siceraria attenuated the extent of acute lung injury induced by oleic acid in rats.
Key Words
Lagenaria Siceraria, lung injury, oleic acid, pneumocyte.
Introduction
Acute respiratory distress syndrome a most severe form of the acute lung injury remains still a major problem with a high mortality in intensive care.
Oleic acid has been generally used to induce acute lung injury in experimental animal models. Septic shock, intestinal ischemia reperfusion, and ventilator induced lung injury models are the other clinically relevant animal models [1]. Oleic acid induced acute lung injury has become one of the most established model of acute lung injury characterized by increased permeability induced pulmonary edema and alveolar infiltration of inflammatory cells [2, 3]. It particularly represents a model of fat embolism syndrome induced acute lung injury [2]. Oleic acid induced lung injury is relatively transient and resolves within hours. It is directly cytotoxic and it was shown that it causes direct inhibition of surfactant function [4].
Lagenaria Siceraria belonging to the family of Cucurbitaceous has been shown to exert antioxidant activity in vitro and in vivo [5, 6, 7]. Traditionally was used for its cardio protective, diuretic, aphrodisiac properties and as antidote to some poisons. It was believed to cure ulcer, fever, asthma, cough and skin diseases [8, 9]. A wide range of chemical compounds such as sterols, terpenoids, flavanoids and saponins have been isolated from the species [10]. LS has been studied to investigate antioxidant, immunomodulatory, analgesic, anti-inflammatory and antihyperglycemic and antihyperlipidemic effects [5].
We aimed to investigate in the current study the effect of LS fruit juice on the oleic acid induced acute lung injury in rats.
Material and Method
Experiments were carried out on 35 female Sprague Dawley rats weighing 250-300 g. The animal care and handling were performed in accordance with the guidelines of the National Institutes of Health. They were housed in polypropylene cages lined with husk renewed every 24 hr, under a 12/12 hr light dark cycle at 22°C and had free access to water. The animals were deprived of food only 12 hours prior to experiment. The protocol of the experiment was approved by Kobay Animal Ethics Committee. The rats were randomized in to 5 groups, containing 7 rats in each. Group I: Control rats received distilled water for 30 days and normal saline (1 ml/kg, sc) Group II: Rats received distilled water 30 days in normal saline and oleic acid 100 mg/kg was applied to the tail vein. Group III: Rats received LS fruit juice and 200 mg/kg/day by gavage for 30 days. Group IV: Rats received LS fruit juice 200mg/kg/day for 30 days by gavage and oleic acid 100 mg/kg was applied to the tail vein. Group V: Rats received LS fruit juice 400 mg/kg /day for 30 days by gavage and oleic acid 100 mg/kg was applied to the tail vein. All the rats were kept in an oxygenated chamber for 4 hours. Rats were anesthetized with intraperitoneal injection of ketamine 80 mg/kg and xzylazine 10 mg/kg. Serum and lung tissue homogenate was used for the estimation of biochemical markers and histopathological examination. Two ml intra cardiac blood was withdrawn 4 hours after oleic acid injection. The whole blood was centrifuged at 3500 rpm for 15 min at 4oC and plasma collected and stored at -80oC. Cytokines (TNFα, IL6, IL10), total tissue oxidant status (TOS) and total tissue antioxidant status (TAS) were measured at serum samples. Then, lung tissue samples were collected and the rats were sacrificed. One lobe of lung was divided into 2 pieces for histopathological exam. One piece was used for light, the other piece was used for electron microscopic examination. One piece of the fresh tissue sample was rapidly fixed in 10% buffered formalin, then dehydrated through graded alcohols and embedded in paraffin blocks. Sections (5 mm) were cut and stained with hematoxylin and eosin (H&E), according to standard protocols. The homogenate of the other piece of lung tissue was centrifuged at 4000 g for 15 min and the supernatant analyzed. Malondialdehyde (MDA), protein carbonyl, catalase, superoxide dismutase (SOD), glutathione peroxide (GPX) was measured in tissue samples.
Collection of Lagenaria siceraria fruits: The fresh fruits of L. siceraria consumed as vegetable by local people were collected in the month of August from the local market of Antakya, Hatay, Turkey and authenticated by the authority of the Department of Horticulture, Faculty of Agriculture, University of Erciyes in Kayseri-Turkey. The fresh juice of LS was prepared with a juicer. Fresh fruit was chopped into small pieces and juice was collected. The juice was filtered with a sterile sponge and filtered part was used for dosing for rats. Ten ml of fresh juice was placed for drying in previously dried and weighed petridish. The juice was evaporated in a autoclave and weight of petridish containing dry residue of juice was taken and equivalent dose of 10 ml juice was calculated by subtracting the initial weight of dried petridish.10 ml juice gives 515 mg total solid residue in dried juice. The dose of fresh juice of LS in ml equivalent to 200 and 400 mg /kg /day was administered by gavage to rats for 30 days in addition to standard diet.
Measurement of cytokines: TNFα, IL6, IL10 levels were evaluated in plasma samples 4 hours after oleic acid injection. The assay was carried out by a colorimetric commercial kit. (Calbiochem-Novabiochem Corp.,San Diego,CA, USA.)
Measurement of Malondialdehyde: The lipid peroxidation product, intestinal tissues were homogenized in 1.15% KCl solution. An aliquot of (100 mcl) of the homogenate was added to a reaction mixture containing 200 mcl of 8.1% sodium dodecyl sulphate 1500 mcl of 20% acetic acid (Ph 3.5), 1500 mcl of 0.8% thiobarbituric acid and 700 mcl distilled water. Samples were then boiled for 1 hr at 95 °C and centrifuged at 3000 g for 10 min. The absorbance of supernatant was measured by spectrophotometry at 650 nm. The results were calculated as nmol.100 mg-1 tissue.
Measurement of SOD: SOD activity was evaluated by inhibition of nitro blue tetrazolium reduction by superoxide anion generated by the xhanthine/xhantineoxide system using a commercial assay kit (Nnjing Jiancheng Biological Product, Nanjing China.) The results were expressed as U.100mg-1 protein.
Measurement of Catalase: Cayman’s catalase assay kit was used to determine the activity of catalase. The method is based on the reaction of the enzyme with methanol in the presence of an optimal concentration of H2O2. The formaldehyde produced was measured in tissue homogenate calorimetrically.
Measurement of GPX: Cayman GPX assay kit was used to measure the activity of GPX. The rate of decrease in the A 340 is directly proportional to the GPX activity in tissue sample.
Measurement of PC: Cayman’s protein carbonyl colorimetric assay kit was used. The amount of protein hydrozone is quantified at an absorbance between 360-385 nm spectrophotometrically. The carbonyl content was standardized to protein concentration.
Measurement of TAS: It was performed using an Aeroset 2.0 analyzer and a total antioxidant status kit. The assay results are expressed as mmol Trolox equivalent/L.
Measurement of TOS:It was performed by using an Aeroset 2.0 analyzer and A TOS kit. The results are expressed as the micro molar hydrogen peroxide equivalent per liter.
Histopathological evaluation
Light microscopic examination: The specimens were examined using a light microscope (Leica DM6000B, Wetzlar, Germany) with a DC490 digital camera (Leica, Wetzlar, Germany) and evaluated for acute lung injury according to the presence of interstitial edema, alveolar hemorrhage, interstitial mononuclear cell infiltration, intraalveolar neutrophils, intraalveolar macrophages and intraalveolar pneumocytes. Each lung specimen was given a score of 0 to 3 depending on whether the finding was absent 0, mild 1, moderate 2, or severe 3. Four randomly selected areas were evaluated under a light microscope (40 X magnification) in each slide by two investigators blinded to the conditions of each animal. The mean value of two investigators’ score was used for graphical and statistical analysis. Total lung injury score was the sum of the average of the score of these six parameters [11].
Electron microscopic examination: The second piece of the fresh tissue samples was fixed in 2.5% glutaraldehyde solution in phosphate buffer, pH 7.4 for 4 h, and post-fixed for 1 h in 1% osmium tetroxide solution in 0.1M phosphate buffer. After washing in phosphate buffer, they were dehydrated in a graded series of ethanol’s to absolute ethanol, treated with propylene oxide and embedded in Araldite/Epon812 (Cat No: 13940, EMS, Hatfield, PA, USA). After heat polymerization, sections were cut using a microtome. Semi-thin sections were stained with methylene blue–azure II and examined using a light microscope (Leica DM6000B, Wetzlar, Germany) with a DC490 digital camera (Leica, Wetzlar, Germany). Ultrathin sections (Leica ultra-cut R, Wetzlar, Germany) were double-stained with urinal acetate and lead citrate (Leica EM AC20). These sections were examined in JEOL-JEM 1400 electron microscope and photographed by CCD camera (Gatan Inc., Pleasanton, CA, USA). The thickness of fused basal lamina in air-blood barrier was measured in 6 different areas in each group and the mean value for each group was calculated.
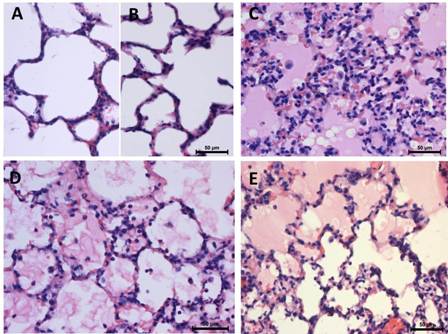
Figure 1: Effect of oleic acid and LS in lung. Normal histological structure of lung with open alveolar spaces, type II and flat type I pneumocytes in group I (A) and group III (B) Hyaline membranes, intraalveoalar inflammatory cells in group II (C) LS in group IV (D) and group V (E) attenuated the lung pathology( Hematoxylin-eosin, X400).
Statistical Analysis
The statistical analysis was performed by using the statistical package for the social sciences version 17, 0 for windows. (SPSS, Chicago, IL) The data are expressed as mean ± Standard Deviation. One way analysis of variance Anova test was used in statistical analysis of parameters, for comparison of the groups. Post-hoc Tukey test was used for secondary comparisons. p<0.05 was considered as statistically significant.
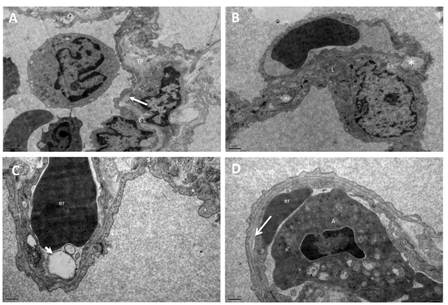
Figure 2: Electron micrographs of the lung from group II. (A) Splitting and lamellation of the basal lamina (arrow), (B) degeneration of mitochondria, and intercellular junctions of type I pneumocyte (asterix), lipid in the cytoplasm of type I pneumocyte. (C) Swelling of the endothelial cells with blebs (arrow head) (D) detachment of the endothelial cells from the basal lamina (arrow) were observed in Group II. BL: fused basal lamina, TP: thick portion of alveolar septum, er: erythrocyte, m: mitochondria, L: lipid, A: apoptotic cells. (A, B: X12 000, C, D: X25 000).
Result
Light microscopic examination of lung tissues in group I and group III revealed normal morphology with open alveolar spaces (Figure 1A, B). Sections of capillaries and flat squamous cells (type 1 pneumocytes); round type II pneumocytes were examined in the alveolar wall. There was no significant difference in the lung injury score between control group and group III. A severe lung injury was determined in group II when compared to group I and group III. Oleic acid resulted in severe pulmonary edema with inflammatory cell infiltration. Moreover per vascular edema thickened alveolar septum, formation of hyaline membranes, and the existence of intraalveolar both inflammatory cells and pneumocytes were examined in group II (Figure 1C). LS fruit juice at a dose of 200 mg/kg and 400 mg/kg reduced lung pathology. Some open alveolar spaces and intraalveolar neutrophil, macrophages and pneumocytes were examined in group IV and group V (Figure 1D, 1E). LS in group III alone didn’t affect the lung injury score.
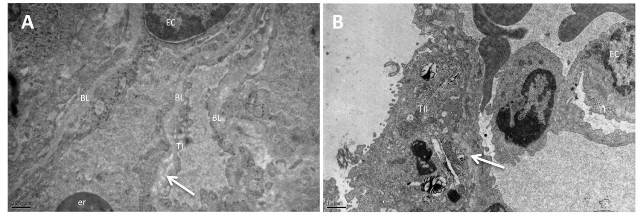
Figure 3: Electron micrographs of the lung from group IV (A), and group V (B) Lamellation and irregularity of the basal lamina (arrow). BL: fused basal lamina, TI: Type I pneumocyte, TII: Type II pneumocyte EC: endothelial cell, er: erythrocyte (A: X25 000, B: X12 000).
In electron microscopic examination of lung sections from group III, thin and thick portions of the alveolar septum revealed normal morphology as group I. The fused basal lamina, endothelial cells and thin cytoplasm of type I pneumocytes was observed in the thin portion of alveolar septum. The collagen fibers were examined in the thick portion. Oleic acid resulted in splitting and lamellation of the basal lamina in the thick portion of alveolar septum (Figure 2A). Degeneration of mitochondria was seen in some Type I pneumocytes. The intercellular junctions were degenerated between pneumocytes (Figure 2B). Welling of the endothelial cells, edema and detachment of the endothelial cells from the basal lamina were observed in some areas (Figure 2C, 2D). The pinocytotic vesicules were prominent in the cytoplasm of type I pneumocytes. The inflammatory cells in the lumen of capillaries were apoptotic with condensed nucleus and widening of the perinuclear sisterna. In some areas degenerated type II pneumocytes were sloughed into the alveolar space in group II.
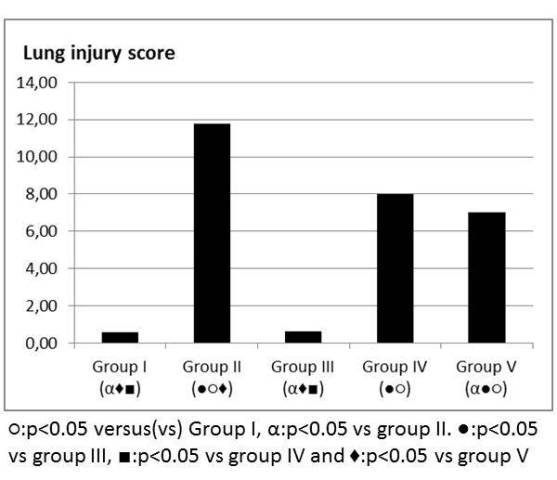
Figure 4: Lung injury score.
LS did not reduce the ultra-structure findings of oleic acid in group IV. Necrotic cells, degenerated type I and type II pneumocytes, lamellation of the basal lamina were observed in group IV. Perinuclear widening was observed in the inflammatory cells in the capillaries and also in the alveolar spaces of group IV. Some endothelial cells also showed perinuclear widening in Group IV (Figure 3A). The mitochondria with their cristae showed normal morphology in the cytoplasm of endothelial cells in Group V. Type I pneumocyte preserved by LS but the lamellation and irregularity of basal lamina remained in group V (Figure 3B).
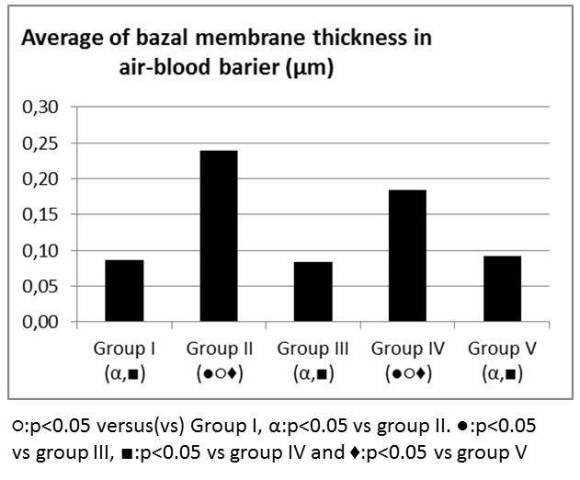
Figure 5: Average of basal membrane thickness in air-blood barrier (µm).
Oleic acid in group II increased the total lung injury score and LS in group IV and V decreased the total lung injury score when compared to group II in light microscopic exam (Figure 4). LS did not change the thickness of the fused basal lamina in air-blood barrier and there was no statistical difference between group I and group III. The thickness of the fused basal lamina was increased in Group II when compared to Group I. LS in group IV and V decreased the thickness of the fused basal lamina when compared to Group II. Decrease in the thickness was more prominent in Group V (Figure 5).
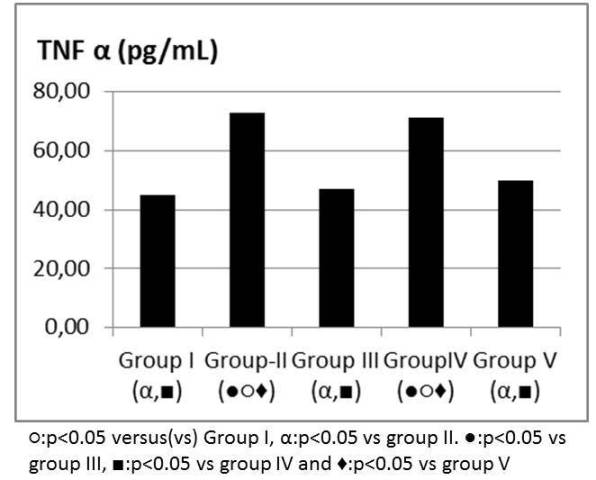
Figure 6: TNF α levels (pg/mL).
TNF alpha, IL6 values were significantly higher in group II when compared to group I and attenuated in group V (Figure 6, 7). IL 10 and TAS, TOS levels were similar between group IV and V when compared to group II (Table 1). MDA was higher in group II when compared to group I and attenuated in group V (Figure 8). SOD, GPX, Catalase and PC levels were not different between groups (Table 2).
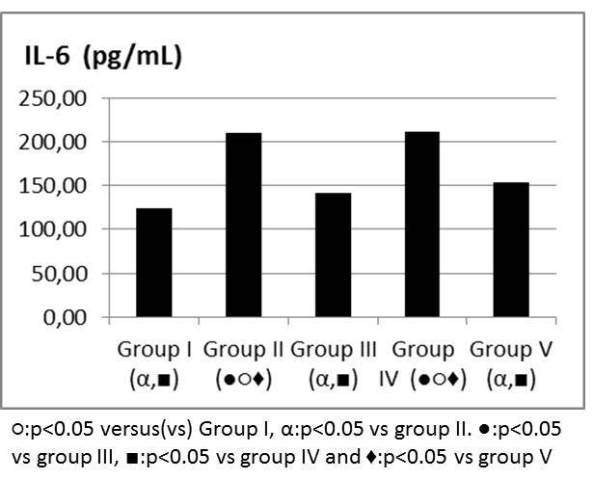
Figure 7: IL-6 levels (pg/mL).
Discussion
It is concluded in the current study that, LS fruit juice at both doses attenuated acute lung injury induced by oleic acid in rats histopathologically. Proinflammatory cytokine and MDA levels were significantly different only in high dose LS group.
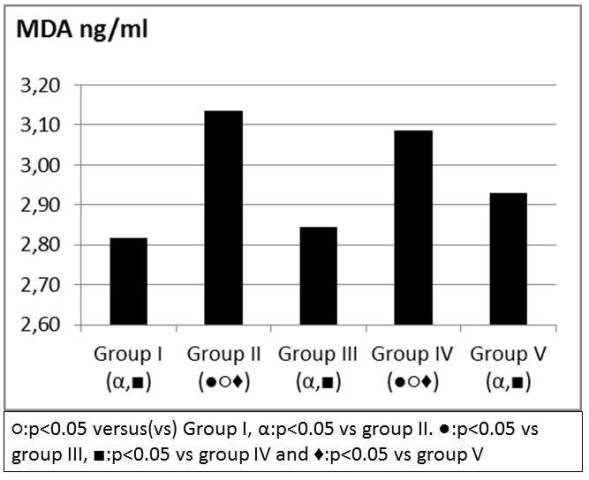
Figure 8: MDA levels (ng/ml).
Acute respiratory distress syndrome was first described by Ashbaugh and Petty in 1967 [12]. Despite improvements in the ventilator strategies and supportive care, mortality of patients with ARDS is very high at 40%-50% [12]. Treatment modalities have been investigated such as using antibodies against inflammatory cytokines, inhibiting fibrin deposition, blocking vasoconstrictors like endothelin, preventing the production of transcription factors and enhancing the alveolar fluid clearance by activating sodium channels [12, 13]. Suppression of the production of TNF alpha or blockade of its biological actions may have a potential therapeutic value against pulmonary diseases characterized by inflammation and cell death [14, 15]. Ito et al showed that ulinastatin, human urinary trypsin inhibitor significantly improved the oleic acid induced histological changes by inhibiting the production of TNF alpha [16]. Mukhopadhyay et al stated that loss of lung surfactant during septic shock may be associated with increased TNF alpha activity [14]. Inhalation of milrinone, which is a PDE 3 inhibitor also has been proposed as a novel strategy for the treatment of acute lung injury [17]. Hit et al found that captopril and other ACE inhibitors may offer a clinic approach for ALI/ARDS treatment since these drugs are widely used in clinical area [1]. Chen et al showed that treatment of propofol attenuated the oleic acid induced inflammatory and pathological changes in conscious rats in acute lung injury model [18].
Table 1: The levels of catalase, GPX, PC, SOD in tissue samples.
|
Group
I
|
Group
II
|
Group
III
|
Group
IV
|
Group
V
|
| Catalase Activity nmol/min/mL |
162,60 |
145,62 |
164,61 |
149,43 |
161,48 |
| Gluthathione peroxide nmol/min/mL |
29,84 |
31,31 |
33,03 |
25,70 |
30,32 |
| Protein Carbonyl (nmol/mL) |
27,50 |
45,52 |
29,22 |
44,55 |
43,38 |
| Superoxide dismutase activity U/mL |
0,63 |
0,54 |
0,54 |
0,60 |
0,71 |
Coagulopathy also plays an important role in the pathogenesis of lung injury [17, 19].It has been shown that as a result of analysis of bronchoalveolar lavage fluid of patients with ARDS/ALI that increased activation of coagulation and inhibition of fibrinolysis correlates with severity of inflammation [17].
Lagenaria siceraria (bottle guard) from the Cucurbitaceous family is a large herb grown throughout the tropical regions of the world [20]. Fard et al demonstrated that LS fruit juice attenuated the doxorubicin induced cardio toxicity by reducing lipid peroxidation in rat heart and preserving endogenous antioxidants [6]. LS fruit juice contains polyphenol compounds. Rajput et al have demonstrated that a flavonol, kaempferol extracted from the fruits of Lagenaria siceraria as a sole agent has been shown to have inherent fibrinolytic activity in vitro [21]. LS was given by gavage to rats for 30 days in this study based on a previous study [6].
Table 2: The levels of IL-10, TAS, TOS in serum samples.
|
Group
I
|
Group
II
|
Group
III
|
Group
IV
|
Group
V
|
| IL-10 pg/dL |
49,67 |
44,99 |
48,36 |
45,99 |
48,21 |
| TAS umol/L |
0,95 |
1,21 |
0,85 |
1,00 |
0,93 |
| TOS mmol/L |
10,28 |
9,05 |
10,73 |
10,96 |
9,36 |
OA induced acute lung injury was characterized by histologically thickening of alveolar septa, alveolar hemorrhage and inflammatory cell infiltration [1, 22]. It causes severe alveolar damage with development of increased permeability alveolar edema [23]. Permeability edema is identified by deregulation of pulmonary epithelial and endothelial apoptosis, liquid clearance capacity of alveolus and integrity of endothelial barrier [24]. Rylader et al speculated that in oleic acid induced lung injury the increase in total lung volume suggesting a predominance of formation of edema over airway and alveolar collapse [3]. Oleic acid model described in the literature varies in terms of animals used, dose of oleic acid, injury time period and use of ventilator support. Only one intravenous injection of oleic acid has been shown to exert its effects on lung within minutes or hours [25]. Mc Guigan et al stated that with the doses of oleic acid they used, the rats did not require ventilator support [25]. We used also single injection of oleic acid at the same dose used in previous study and did not ventilate the rats. We used anesthetic agents only for ethical reasons. Histopathological changes induced by oleic acid are similar to the exudative phase of ARDS which is characterized by interstitial and intraalveolar edema, intraalveolar hemorrhage and vascular congestion and intravascular coagulation [25, 26]. Our findings were in accordance with the literature.
The role of free radicals in oleic acid induced acute lung injury is controversial in the literature [25, 27]. Liu et al showed that reactive oxygen species increased immediately after of oleic acid injection and reached to its peak value in 30 minutes [27]. Oxidative injury to the pulmonary capillary endothelial cells might be the first step in initiation of acute lung injury induced by oleic acid [27]. On contrary to this, McGuigan et al demonstrated that free radicals do not contribute to the initial stages of oleic acid induced lung injury as a result of ethidium superoxide assay up to 8 hours [25]. It would be more likely to find the free radicals with a longer duration of injury [25]. We found in our study that MDA level was attenuated. LS might show its protective effect against oleic acid induced acute lung injury by preventing oxidative stress.
One limitation of our study that the difficulty of the result of oleic acid induced acute lung injury that should be cautiously extrapolated to human ARDS. Another limitation is LS was administered before the onset of ARDS, whereas ARDS recognition and treatment were delayed clinically. Risk factors of patients for ARDS could be identified and these patients might be treated prophylactically in intensive care setting.
Future studies are required in order to define the optimal pharmacotherapy for ALI/ARDS and LS fruit juice might be a part of early and appropriately provided enteral nutrition to critically ill patients at risk for ARDS.
Author’s Contribution
AES: Planning, collecting data, writing of manuscript.
FY: Planning, collecting data, statistical analysis of manuscript.
IOO: Collection data of manuscript.
DZ, FFK: Histological examination.
MA: Planning of manuscript.
AD, SU: Evaluation of data.
HY: Writing and evaluation of data.
MK: Planning, Evaluation of data.
Conflicts of Interest
The author declares there are no conflicts of interest.
Ethical Considerations
Kobay Animal Research Labaratory.No:30-22.11.2011.
Funding
Etlik Research and Trainning Hospital Research Funding
Acknowledgement
None
References
[1]. He X, Han B, Mura M, Xİa S, Wang S, Ma T et al. Angiotensin converting enzyme inhibitor captopril prevents oleic acid induced severe acute lung injury in rats.Shock 2007;28:106-11. [Pubmed].
[2]. Kennedy MT, Higgins BD, Costello JF, Curtin WA, Laffey JG. Hypertonic saline reduces inflammation and enhances the resolution of oleic acid induced acute lung injury. BMC Pulmonary Medicine 2008;8:9 doi:10.1186/1471-2466-8-9. [Pubmed].
[3]. Rylander C, Hogman M, Perchiazzi G, Magnusson A, Hedestierna G. Oleic acid lung injury:a morphometric analysis using computed tomography. Acta Anaesthesiol Scand 2004;48:1123-9. [Pubmed].
[4]. Dirocco JD, Pavone LA, Carney DE, Lutz CJ, Gato LA, Landas SK. et al. Dynamic alveolar mechanics in four moels of lung injury. Intensive Care Med 2006;32:140-8. [Pubmed].
[5]. Kubde MS, Khadabadi SS, Farooqui IA, Deore SL. Lagenaria siceraria:Phytochemistry, pharmacognosy, and pharmacological studies. Report and Opinion.2010;2:91-8.
[6]. Fard MH, Naseh G, Bodhankar SL,Dikshit M. Cardioprotective effect of Lagenaria Siceraria Standley fruit juice on doxorubicin induced cardiotoxicity in rats. Am J Pharmacol and Toxicol 2010; 5:103-8.
[7]. Vijayakumar M, Selvi V, Krishnakumari S. Cardioprotective effect of Lagenaria siceraria ameliorates isoproterenol induced cardiac toxicity in rats by stabilizing cardiac mitochondrial and lysosomal enzymes. Toxicological and Environmental Chemistry.2011;93:171-6.
[8]. UA1. Upaganlawar A, Balaraman R. Protective effects of Laginaria Sceraria fruit juice in ısoproterenol induced myocardial infarction. Int J Pharmacol 2010;6:645-51.
[9]. Jadhaw TA, Joshi YM, Kadam VJ. In vitro antioxidant activity of ethanolic extract of the leaves of Lagenaria siceraria. Journal of Pharmacy Research 2010;3:257-60.
[10]. Prajapati RP, Kalariya M, Parmar SK, Sheth NR Phytochemical and pharmacological review of Lagenaria sicereria. J Ayurveda Integr Med. 2010 Oct;1(4):266-72. [Pubmed].
[11]. RS Higgins, GV Letsou, JA Sanchez, RN Eisen, GJ Smith, KL Franco et al. Improved ultrastructural lung preservation with prostaglandin E1 as donor pretreatment in a primate model of heart-lung transplantation. J Thorac Cardiovasc Surg 1993;105: 965-71. [Pubmed].
[12]. Jain R, DalNogare A. Pharmacological Therapy for acute respiratory distress syndrome. Mayo Clin Proc.2006;81:205-12. [Pubmed].
[13]. Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Noter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem 2008;15:1911-24. [Pubmed].
[14]. Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNF alpha in pulmonary pathophsiology. Respiratory Research.2006;7:125. doi:10.1186/1465-99217-125. [Pubmed].
[15]. Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine mediated inflammation in acute lung injury. Cytokine and Growth factor reviews 2003;14:523-35. [Pubmed].
[16]. Ito K, Mizutani A, Kira S, Mori M, Iwasaka H, Noguchi T. Effect of ulinastatin, a human urinary trypsin inhibitor, on the oleic acid induced acute lung injury in rats via the inhibition of activated leucocytes. Injury 2005;36:387-94. [Pubmed].
[17]. BueltmannM, Kong X, Mertens M, Yin N, Liu Z, Koster A et al. Inhaled milrinone attenuates experimental lung injury. Intensive Care Med 2009;35:171-8. [Pubmed].
[18]. Chen HI, Hsieh NK, Kao SJ, Su CF. Protective effects of propofol on acute lung injury induced by oleic acid in concious rats.Crit care med 2008;36:1214-21. [Pubmed].
[19]. Schultz MJ, Haitsma JJ, Zhang H, Slutsky AS. Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia-A review. Crit Care Med 2006;34:871-7. [Pubmed].
[20]. Kalsait RP, Khedekar PB, Saoji AN, Bhusari KP. Isolation of phytoserols and antihyperlipidemic activity of Lagenaria siceraria. Arch Pharm Res. 2011;34:1599-604. [Pubmed].
[21]. Rajput MS, Mathur V, Agrawal P, Chandrawanshi HK, Pilaniya U. Fibrinolytic activity of kaempferol isolated from the fruits of Lagenaria siceraria Standley. Nat Prod Res 2011;25:1870-5. [Pubmed].
[22]. Wang HM, Bodenstein M, Markstaller K. Overview of the pathology of three widely used animal models of acute lung injury.Eur Surg Res 2008;40:305-16. [Pubmed].
[23]. Zhou Z, Kozlowski J, Schuster DP. Physiologic, biochemical, and imaging characterization of acute lung injury in mice. Am J Respir Crit Care Med 2005;172:344-51. [Pubmed].
[24]. Lucas R, Verin AD, Black SM, Catravas JD. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochemical Pharmacology 2009;77:1763-72. [Pubmed].
[25]. McGuigan RM, Mullenix P, Norlund LL, Ward D, Walts M, Azarow K. Acute lung injury using oleic acid in the labaratory rat: Establishment of a working model and evidence against free radicals in the acutye phase. Current Surgery 2003;60:412-7. [Pubmed].
[26]. Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 2006;27:337-49. [Pubmed].
[27]. Liu H, Zhang D, Zhao B, Zhao J. Superoixide anion , the main species of ROS in the development of ARDS induced by oleic acid. Free Radical Research.2004;38:1281-7. [Pubmed].

