
Transformation of recurrent fibroadenoma to phyllodes tumour: a case report and review of literature
Kartikeyan Senniappan1, Navin Sharma1, Devendra Kumar Ravi1, Mohan Kumar2, Mridula Shukla2, Manoj Pandey1
- 1Departments of Surgical Oncology, Institute of Medical Sciences, Banaras Hindu University, Varanasi India
- 2Departments of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi India
- Submitted: February 6, 2012,
- Accepted June 12, 2012,
- Published June 23, 2012
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Introduction:
Fibroadenoma, though considered a common benign breast lesion, can undergo malignant changes, majority of which are in situ carcinomatous lesions..
Case report:
We report one such case and discuss the possible genetic reason for this change. The clinical and radiological features of these tumours, diagnostic pathological tools, their limitations and the treatment modalities are also discussed.
Conclusions:
Fibroadenoma transforming into phyllodes tumour is a very rare phenomenon Only six cases have been reported in the literature previously.
Introduction
Fibroadenoma is a common benign breast lesion that typically occurs in young patients between ages of 20 and 35 years. It is a fibro-epithelial lesion consisting of both epithelial and stromal elements. Though considered as a benign proliferative breast disease, malignant changes in fibroadenoma have been documented in 0.1% of the cases [1,2,3], which usually involve epithelial components and the large majority of these are in situ lesions [4,5]. Fibroadenoma transforming to phyllodes tumour is a very rare phenomenon with three cases reported by Noguchi
et al., [6] and single case reported by Edna K Valdes et al., [7] Pacchiarotti A
et al.,[8] and Hodges KB et al., [9] each. We report one case of recurrent fibroadenoma of breast progressing to phyllodes tumour on its second recurrence.
Case report
A 38-year-old woman presented with complaint of slowly progressive lump in the left breast of 5-year duration. There was no associated pain or nipple discharge. On examination a 10x6cm bosselated swelling with variegated consistency was present in upper quadrant. There were no axillary nodes and opposite breast and axilla were normal. Fine needle aspiration cytology (FNAC) was carried out that showed a cellular aspirate composed of benign ductal cells in cohesive groups and in papillary clusters, bare nuclei and myoepithelial cells, a few ductal cells showed atypical features. The aspirate was consistent with benign proliferative disease of breast. Mammogram showed a huge lobulated opacity with well-defined margins and no calcification. Features were suggestive of giant fibroadenoma. A wide excision was done. On cut opening the specimen a 10x6x4 cm well encapsulated mass was found. Frozen section was carried out which was inconclusive. On examination of paraffin sections the neoplasm was composed of proliferated ducts and acini set in a fibro myxoid / fibro collagenous stroma. Elongated, compressed and distorted ducts were seen in areas (Figure 1). Marked adenosis with proliferated tubules lined by inner epithelial and outer myoepithelial cell layers were seen. There was peritubular hyalinisation. The microscopy was suggestive of fibroadenoma with adenosis.
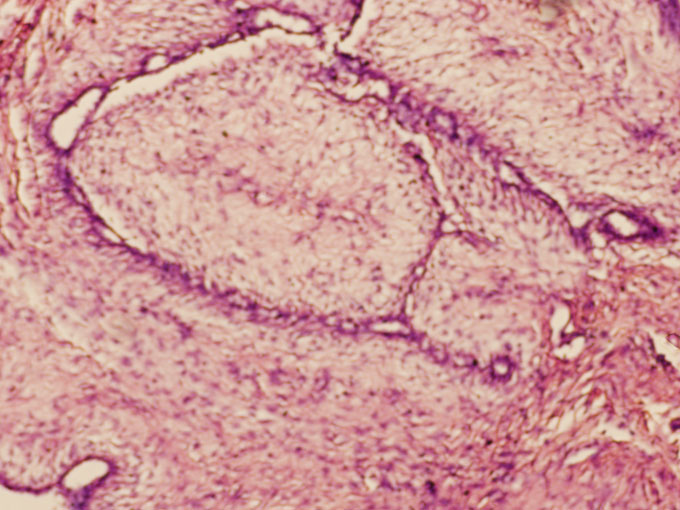
Figure 1: Photomicrograph showing fibroadenoma
Three years later in 2000 patient presented with a rapidly progressive lump in the same breast of two months duration. On examination a 6x5cm swelling was noted in upper outer quadrant beneath the scar. There were no axillary nodes. FNAC from the swelling showed only benign ductal cells. A wide excision was carried out. Resected specimen on
cut section showed 8.5x7cm neoplasm with glistening white cut surface. A frozen section was carried out which showed dilated and elongated ducts in a cellular spindle cell stroma. The features were suggestive of phyllodes tumour; however a definite opinion could not be given. Examination of paraffin sections showed a neoplasm composed of proliferated ducts and acini with fibro collagenous stroma which appeared cellular with plum
cells and occasional mitotic figures a diagnosis of fibroadenoma was given.
Patient was disease free for 2-years when in 2002 she developed recurrent lump in same breast at same location. The lump was progressively enlarging to occupy almost whole breast within one month. There was no pain, nipple discharge or axillary nodes. There was involvement of skin and surrounding inflammation. Patient was taken up for wide excision which amounted to almost a simple mastectomy. As the skin could not be closed primarily a LD flap reconstruction was carried out. Examination of resected specimen revealed a liner healed scar 16 cm long and a large 19x16x19 cm tumour was seen beneath the scar. On microscopy the neoplasm was composed of spindle shaped cells, with mildly pleomorphic short spindle nucleus, eosinophilic cytoplasm and indistinct cell margins, arranged in fascicles and loose sheets, in a myxoid stroma. Stroma showed 4-5 mitoses/ 10 HPF along with thin walled vascular channels and mild infiltration by lymphocytes and
mast cells (Figure 2). A diagnosis of phyllodes tumour-intermediate grade was given.
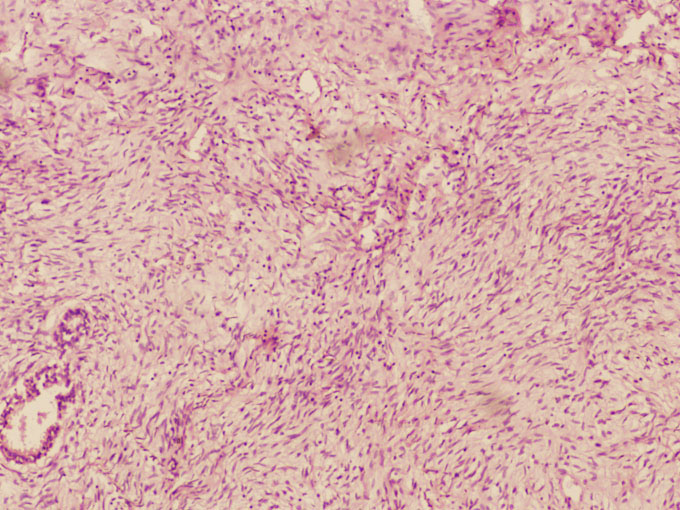
Figure 2: photomicrograph showing phyllodes tumor
The case was discussed in the breast clinic and in view of clear margins and intermediate grade it was decided to keep over on regular follow-up only without any adjuvant treatment. She is disease free and on regular follow-up.
Discussion
Fibroadenoma and phyllodes tumour are neoplasms arising form intralobular stroma of breast [10]. Though they are stromal lesions they contain epithelial elements also and hence termed fibro epithelial lesions of breast. Many pathologists consider these lesions as a continuum of spectrum of disease with fibroadenoma at the benign end and malignant phyllodes at the other end with borderline phyllodes in between. Abe M
et al., reported 11 cases that were misdiagnosed as fibroadenoma and later recurred and were diagnosed as phyllodes on recurrence. They found that the prognosis of these patients was better than those diagnosed primarily as phyllodes tumor [11].
The importance of distinguishing fibroadenoma from phyllodes tumour resides in the fact that fibroadenoma can be observed clinically after diagnosis where as the treatment of phyllodes is a wide excision with a minimum 1 cm of normal tissue margin.
Differentiating fibroadenoma from low grade phyllodes (benign/borderline) is quite challenging. Clinically fibroadenoma occurs in young women with average age of 20-30 years [12] where as phyllodes tumour occur in women aged 30-55 years, average age being 40 years [12,13], however it can occur even at younger age and in adolescents [14]. Though both of these present as a firm, painless, smooth, well circumscribed and mobile mass; phyllodes tumour is usually rapidly growing to attain larger size at presentation and occasionally causes pressure necrosis of overlying skin.
Many of the phyllodes tumours cannot be distinguished from fibroadenoma on radiological investigations [15] with phyllodes tumour often mimicking fibroadenoma at mammography [16], commonly appearing as a large, well circumscribed oval or lobulated mass with rounded borders [17] which may show a lucent halo or coarse micro calcifications [18]. Ultra sonogram typically shows an inhomogeneous solid appearing hypoechoeic mass with smooth walls. Identification of a cyst within a solid lesion by ultra sonogram is highly suggestive of phyllodes tumour [16].
Pathologically, though fibroadenoma and phyllodes tumour both have a dimorphic pattern with epithelial and stromal components, the key diagnostic features lie in the stromal components with phyllodes tumour having increased cellularity, pleomorphism, nuclear atypia and mitotic figures [12,19]. The value of fine needle aspiration cytology in diagnosis of phyllodes remains controversial with an overall accuracy ranging from 63% [20] to 92.8% [21]. If diagnosis is in doubt, core needle biopsy of the lump is advised [20], which can easily differentiate malignant phyllodes tumour, with features of marked increased stromal cellularity, marked nuclear pleomorphism and atypia, marked stromal overgrowth, infiltrative borders and high mitotic rate [22]. The biopsy features like stromal cellularity being increased in at least 50% in comparison with normal fibroadenoma, stromal overgrowth at 10x magnification, fragmentation and presence of adipose tissue within the lesion, can distinguish the low grade phyllodes tumour (benign/borderline) from fibroadenoma thereby helping in treatment decisions [23].
The phyllodes tumour can be categorised according to the mitotic figures as benign, borderline or malignant based on the classification by Norris and Taylor [24]. The lesions with 0-4 mitoses/10hpf with minimal atypia was classified as benign, 5-9 mitoses/10hpf with moderate atypia as borderline and
>10 mitoses / 10hpf with marked atypia as malignant.
Flow cytometric analysis of markers of cell proliferation like S-phase fraction, DNA ploidy, p53 expression and MIB-1 index, have been used to assess the tumour grade and to predict clinical outcome in situations of diagnostic dilemma to aid in clinical decision making. These are not used on routine basis due to high costs involved and varied interpretation of results due to lack of definitive cut off values for each of these factors [20].
Noguchi et al., [6] in their study of progression of fibroadenoma to phyllodes tumour, suggested that from the clonal point of view there are two types of fibroadenoma, the much common polyclonal type and the rare monoclonal tumour. They postulated that a polyclonal fibroadenoma can turn into a monoclonal tumour due to mutations in any single stromal cell which then undergoes rapid multiplication and progression to phyllodes tumour. Thus PCR method for clonal analysis can be done on FNA samples of large, rapidly growing suspicious fibroadenomas to establish the mono or poly clonal nature of the tumour to aid in treatment decisions, as the monoclonal fibroadenoma can progress to or recur as phyllodes tumour later and hence should be treated like phyllodes tumour with a wide excision at the onset. This monoclonality could have well been the reason in our case of fibroadenoma recurring as phyllodes tumour. Allelic loss at D7S522 has been demonstrated in both fibroadenoma and phyllodes tumors. In addition, phylloid tumor also show allelic loss at TP53 and D22S264, that is not observed in fibroadenoma, suggesting that these tumors are clonally related and allelic loss at TP53 and D22S264 may be implicated in the progression of FA to phyllode tumor [9].
The effort in distinguishing fibroadenoma from phyllodes tumour is worthwhile for the reason that fibroadenoma after diagnosis can be followed up but the phyllodes tumour should be excised with a margin of at least 1 cm of normal breast tissue. Core cut biopsy is potentially the most useful investigation, when clinical suspicion of phyllodes tumour is raised due to features like
>3 cm size, sudden increase in size, age >35 years and suspicious radiological features with inconclusive FNAC .
It is also important to know that a biopsy diagnosis of fibroadenoma does not completely exclude the diagnosis of phyllodes tumour because of heterogeneity of phyllodes tumour with less cellular areas resembling fibroadenoma also present within the tumour.
Treatment of phyllodes tumour is wide local excision with 1-2 cm normal tissue margin or a simple mastectomy if tumour is large and occupying the whole breast tissue [25]. Lymphatic involvement is considered rare [13] and hence axillary lymph node dissection is not recommended, unless they are clearly involved with tumour [22]. The presence of metastatic disease in the nodes should be confirmed prior to nodal dissection. Approximately one third to one half of malignant phyllodes tumours will metastasize, mainly by haematogenous route to lung (70-80%) followed by bone (25-30%). Local recurrence occurs in 5-15% of benign tumours and 20-30% of malignant cases [24].
The role of adjuvant radiotherapy is not well established. August et al., [13] suggested radiotherapy after wide local excision for high risk lesions (tumour
>5cms, presence of stromal overgrowth, >10 mitoses/10hpf, infiltrative margins), for local recurrence would likely be avoided in 90% or more of these patients [26]. The role of chemotherapy seems to be limited to the treatment of metastases and for palliation of unresectable local recurrences [27].
Follow-up for malignant phyllodes tumour consists of biannual physical examination for 5 years, then annually; baseline unilateral mammogram 3 months post excision with or without radiotherapy should be done if breast was conserved, followed by annual bilateral screening mammography; biannual CT of chest to be performed for 2-5 years for high risk lesions [27].
Conclusion
The present article emphasises the difficulty in differentiating phyllodes tumour from the fibroadenoma and the importance of recently developed [20,23] reliable and reproducible pathological features in the core cut biopsy specimens for definitive pre-operative diagnosis is stressed. Finally, it is important to remember that a core biopsy diagnosis of fibroadenoma does not completely exclude the diagnosis of phyllodes tumour owing to its heterogeneity, thereby highlighting the importance of clinical judgement in these tricky fibro epithelial lesions.
Authors’ Contribution
KS: did the literature search and prepared the draft manuscript
NS: Helped in preparation of draft manuscript
DKR: Did the literature search and prepared the manuscript
MK: Contributed to the pathological part of the manuscript
MS: wrote the pathological part of the manuscript
MP: Conceived and designed the study and edited the final version
All authors have read and approved the manuscript
Ethical consideration
Patients consent was obtained for reporting this case
Conflict of interest
The authors declare that there are no conflicts of interests
Funding Source
None
References
[1]. Buzanowski-Konakry K, Harrison EG Jr, Payne WS. Lobular carcinoma arising in fibroadenoma of the breast. Cancer 1975; 35: 450-6. [Pubmed]
[2]. Goldman RL, Friedman NB. Carcinoma of breast arising in fibroadenomas with emphasis on lobular carcinoma. A clinicopathologic study. Cancer 1969;23:544-550.[Pubmed]
[3]. McDivitt RW, Stewart FW, Farrow JH. Breast carcinoma arising in solitary fibroadenomas. Surg Gynecol Obstet 1967;125:572-576.[Pubmed]
[4]. Diaz NM, Palmer JO, McDivitt RW. Carcinoma arising within fibroadenomas of breast. A clinicopathologic study of 105 patients. Am J Clin Pathol 1991;95:614-622.[Pubmed]
[5]. Fondo EY, Rosen PP, Fracchia AA, Urban JA. The problem of carcinoma developing in a fibroadenoma. Recent experience at Memorial Hospital. Cancer 1979; 43: 563-567.[Pubmed]
[6]. Noguchi S, Yokouchi H, Aihara T, Motomura K, Inaji H, Imaoka S, Koyama H. Progression of fibroadenoma to phyllodes tumour demonstrated by clonal analysis. Cancer 1995; 76: 1779-85.[Pubmed]
[7]. Valdes EK, Boolbol SK, Cohen JM, Feldman SM. Malignant transformation of a breast fibroadenoma to cystosarcoma phylloides. The Am Surg 2005; 71(4): 348-353.[Pubmed]
[8]. Pacchiarotti A, Frati P, Caserta D, Pacchiarotti A, Frega A, Moscarini M. First case of transformation for breast fibroadenoma to high-grade malignant cystosarcoma in an in vitro fertilization patient. Fertil Steril. 2011 Nov;96(5):1126-7.[Pubmed]
[9]. Hodges KB, Abdul-Karim FW, Wang M, Lopez-Beltran A, Montironi R, Easley S, Zhang S, Wang N, MacLennan GT, Cheng L. Evidence for transformation of fibroadenoma of the breast to malignant phyllodes tumor. Appl Immunohistochem Mol Morphol. 2009 Jul;17(4):345-50.[Pubmed]
[10]. Kumar V, Abbas K, Fausto N. Robbins and Cotran Pathologic basis of disease; 7th edition 2004 Saunders, Philadelphia 1149-1150.
[11]. Abe M, Miyata S, Nishimura S, Iijima K, Makita M, Akiyama F, Iwase T. Malignant transformation of breast fibroadenoma to malignant phyllodes tumor: long-term outcome of 36 malignant phyllodes tumors. Breast Cancer. 2011 Oct;18(4):268-72.[Pubmed]
[12]. Noguchi S, Motomura K, Inaji H, Imaoka S, Koyama H. Clonal analysis of fibroadenoma and phyllodes tumour of the breast. Cancer Res 1993; 53: 4071-4. [Pubmed]
[13]. August DA, Kearney T. Cystosarcoma phylloides: mastectomy, lumpectomy or lumpectomy plus irradiation. Surg Oncol 2000; 9: 49-52.[Pubmed]
[14]. Rajan PB, Cranor ML, Rosen PP. Cystosarcoma phylloides in adolescent girls and young women. A study of 45 patients. Am J Surg Pathol 1998;22:64-69.[Pubmed]
[15]. Page JE, Williams JE. The radiological features of phylloides tumour of the breast with clinico-pathological correlation. Clin Radiol. 1991; 44(1): 8-12.[Pubmed]
[16]. Jorge Blanco A, Vargas Serrano B, Rodríguez Romero R, Martínez Cendejas E. Phyllodes tumours of breast. Eur Radiol 1999; 9: 356-60.[Pubmed]
[17]. Feder JM, de Paredes ES, Hogge JP, Wilken JJ. Unusual breast lesions: radiologic-pathologic correlation. Radiographics. 1999; 19 Spec No:S11-26; quiz S260.[Pubmed]
[18]. Cosmacini P, Zurrida S, Veronesi P, Bartoli C, Coopmans de Yoldi GF.. Phyllode tumor of the breast: mammographic experience in 99 cases. Eur J Radiol. 1992;15(1):11-4.[Pubmed]
[19]. Geisler DP, Boyle MJ, Malnar KF, McGee JM, Nolen MC, Fortner SM, Broughan TA. Phyllodes tumors of the breast: a review of 32 cases. Am Surg 2000;66:360-6.[Pubmed]
[20]. Jacklin RK, Ridgway PF, Ziprin P, Healy V, Hadjiminas D, Darzi A. Optimising preoperative diagnosis in phyllodes tumour of the breast. J Clin Pathol 2006; 59: 454-459.[Pubmed]
[21]. Jayaram G, Sthaneshwar P. Fine-needle aspiration cytology of phyllodes tumors. Diagn Cytopathol 2002;26:222-7.[Pubmed]
[22]. Pietruszka M, Barnes L. Cystosarcoma phyllodes: a clinicopathologic analysis of 42 cases. Cancer 1978; 41:1974–83.[Pubmed]
[23]. Lee AH, Hodi Z, Ellis IO, Elston CW. Histological features useful in the distinction of phyllodes tumour and fibroadenoma on needle core biopsy of the breast. Histopathology 2007; 51: 336–344.[Pubmed]
[24]. Norris HJ, Taylor HB. Relationship of histologic features to behavior of cystosarcoma phyllodes. Analysis of ninety-four cases. Cancer 1967; 20: 2090–9. [Pubmed]
[25]. Chaney AW, Pollack A, McNeese MD, Zagars GK, Pisters PW, Pollock RE, Hunt KK. Primary treatment of cystosarcoma phyllodes of the breast. Cancer 2000; 89: 1502-11.[Pubmed]
[26]. Harada S, Fujiwara H, Hisatsugu T, Sugihara H. Malignant cystosarcoma phyllodes with lymph node metastasis; case report. Jpn J Surg 1987;17:174-7.[Pubmed]
[27]. Pandey M, Mathew A, Kattoor J Abraham EK, Mathew BS, Rajan B, Nair KM. Malignant phyllodes tumor. Breast J 2001;7:411-6. [Pubmed]
[28]. Kapiris I, Nasiri N, A'Hern R, Healy V, Gui GP. Outcome and predictive factors of local recurrence and distant metastases following primary surgical treatment of high-grade malignant phyllodes tumours of the breast. Eur J Surg Oncol 2001; 27: 723-30.[Pubmed]

