Research

Expression of Caspase 3 and Inhibition of Apoptosis Lowers Survival in Breast Cancer
1Kumkum Jha,2Mridula Shukla 2Mohan Kumar 3Vijay K Shukla, 1Manoj Pandey
- 1Departments of Surgical Oncology
- 2Departments of Pathology
- 3General Surgery Institute of Medical Sciences Banaras Hindu University Varanasi 221 005, India
- Submitted: Monday, August 7, 2017
- Accepted: Monday, August 21, 2017
- Published: Thursday, August 31, 2017
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Background
Breast epithelial cell homeostasis requires the balance between cell proliferation and cell death. Apoptosis is induced by two different mechanisms: the extrinsic or receptor- dependent pathway and the intrinsic or mitochondria dependent pathway. One of the key mediators of apoptosis is the thiol protease caspase 3. In this study we report the mRNA and protein expression of caspase 3 in breast cancer and its relationship with clinico-pathological parameters, survival and apoptosis.
Materials and Methods
Caspase 3 expression was measured by immunohistochemistry, western blotting and RT PCR in 90 breast cancer and 29 controls . Apoptosis was detected by in situ labelling of the fragmented DNA (terminal deoxynucleotidyl transferase mediated UTP nick end-label (TUNEL) staining).
Results
Caspase 3 protein expression was found in 69% of controls and 48.9% of breast cancer cases. The mRNA was expressed in 38.9% of breast cancer and 62.1% of controls. Significantly higher caspase 3 mRNA expression was seen in breast cancer. Caspase 3 expression showed significant association with with tumour stage, metastasis and apoptosis. Higher over all 3 year survival was seen in caspase 3 and apoptosis negative breast cancer however the difference was not significant.
Conclusions
A significantly lowered caspase 3 expression in breast cancer compared to benign tumours was observed. However, there was no significant difference in survival between breast cancer patients expressing caspase and those who did not. Presence of metastasis and oestrogen receptor positivity was found to be independent prognostic factors in multivariate analysis.
Introduction
In breast cancer apoptosis can be detected by counting the apoptotic cells in conventional histopathological sections [1] or by using special staining techniques, which are based on in situ labelling of the fragmented DNA (terminal deoxynucleotidyl transferasemediated UTP nick end-label (TUNEL) staining) [2-5] or by studying caspase cascade. Caspases are a group of cysteine proteases that play a central role in both apoptotic initiation and execution because of their ability to activate proteins via a complex system of direct and feedback loops [6]. To date, 14 mammalian caspases have been cloned. These have been subdivided into three major groups based on functional similarities: i) initiator or apical caspases, such as caspase-2, -8, -9 and -10; ii) effector caspases, such as caspase-3, -6 and -7; and iii) caspases involved in cytokine maturation and inflammation, such as caspase-1, -4 and -5 [6, 7].
The regulation of caspases is believed to be primarily at the protein level. In the absence of an apoptotic signal, caspase proteins are inactive zymogens, or procaspases. Proapoptotic signals activate caspases by cleavage of these proteins, resulting in functional enzymes [8]. Activation of the caspase cascade eventually results in proteolysis of numerous subcellular substrates, such as poly-(ADP-ribose) polymerase (PARP), and Lamin B. Alterations of caspase expression appear to be a factor contributing to aberrant apoptosis in cancer cells.
Studies of human breast cancers indicate that this may be an important and frequent phenomenon, as it accounts for approximately 75% of the tumours that lack caspase-3 transcription and protein expression [9]. Caspase 3 has also been shown to facilitate radiation induced and chemical carcinogenesis and genome instability in cell culture studies [10]. It has been hypothesized that caspase deficiency/down-regulation may be common in breast cancer and is likely to enhances cell survival, even in the setting of significant cell aberrations, thus promoting breast carcinogenesis and therapeutic resistance [9].
In the present study, expression of caspase 3 and its relation with apoptosis, clinico-pathological parameters, socio-demographic parameters and prognosis was studied.
Materials and methods Patients
Materials and methods PatientsAfter obtaining a written informed consent tissues were obtained from 90 patients of breast cancer cases and 29 benign breast diseases (fibroadenoma) attending the Surgical Oncology and General Surgery outpatient, and scheduled to undergo surgery as part of their treatment. The study was approved by the institute ethics committee. After collection, part of the samples was stored in RNA latter for mRNA study, part snapped freeze in liquid nitrogen for protein study and later stored at -84 °C till further processing. A portion of the sample was stored in buffered formalin for histology and immunohistochemistry.
Immunohistochemistry
Four micron sections were prepared on poly L-lysine coated glass slides from the formalin fixed paraffin embedded tissues. Sections were transferred to xylene with three changes of 5 min each. Rehydration was done with graded alcohol from 100% to 70% followed by running tap water for 30 min. Antigen retrieval was done in citrate buffer for 20 min. Slides were washed 3 times after retrieval in TBS for 10 minutes each. Endogenous peroxidise activity was blocked by incubating section with 0.3% peroxide (H202) in methanol for 20 minutes. Sections were washed in TBS for 5 minutes and were incubated in normal blocking serum for 1 hour to block non-specific binding sites. Slides were incubated overnight with Caspase 3 antibody (caspase-3 (3CSP03): sc-56046, Santa Cruz Biotechnology, Inc.). Slides were washed in TBS for 5 minutes. Secondary staining was done as per manufacturer’s instruction using Vectastain Universal Elite Abc Kit from Vector laboratories (Cat.No.PK-6200). Slides were washed for 5 minutes in TBS. Sections were incubated in peroxidise substrate solution for colour development. Finally sections were counterstained with haematoxylin for observation under light microscope for image analysis and quantification.
Grading of immunostaining
Immunostaining intensity was graded as Strong (+++), Moderate (++), Weak (+) or nil (0) staining.
RT-PCR
Total RNA was extracted by TRIZOL (Invitrogen) and reverse transcribed to cDNA with High Capacity cDNA Reverse Transcription Kits (Applied Biosystem). PCR reactions were performed using GAPDH as an internal standard. Primers used were: Caspase 3 forward: 5’- TTAATAAAGGTATCCATGGAGAACACT-3’,Caspase 3 Reverse 5’- TTAGTGATAAAAATAGAGTTCTTTTGTGAG -3’. The predicted length of the amplified product was 849bp [9]; GAPDH forward primer (FP): 5’-ACGGATTTGGTCGTATTGGGCG-3’, GAPDH reverse primer (RP) 5’-CTCCTGGAAGATGGTGATGG-3’. The predicted length of that amplified product was 212 bp [10]. The PCR cycles were set for total 33 cycles with denaturation temperature at 94 ℃ for 30 seconds, annealing temperature at 54 ℃ for 40 seconds and extending temperature at 72 ℃ for 1 min per cycle and last cycle lasted for 10 min at 72 ℃.
Western Blotting
Tissue were diluted in sodium dodecyl sulphate (SDS) buffer containing 50mM Tris-HCL (pH 6.8), 4% beta mercaptoethanol, 4% SDS, 20% glycerol and 0.01% bromophenol blue. Protein from each sample was separated on a 12% SDS–polyacrylamide gel. Following electrophoresis, the separated proteins were transferred to a PVDF transfer membrane (Thermoscientific) using a wet blotting apparatus for overnight. The membranes were then treated for 1 hour at room temperature with a blocking solution containing 50mM Tris buffered saline (TBS), 5% skimmed milk powder and 0.05% Triton-X-100 (TBS-T).Following this, the membranes were incubated overnight at 4ºC with caspase 3 antibody (caspase-3 (3CSP03): sc-56046, Santa Cruz Biotechnology, Inc), in a blocking solution containing 50mM TBS-T and 5% skimmed milk powder. Subsequently, membranes were washed in 50mM TBS containing 0.05% Triton and then probed with Goat anti mouse IgG-HRP (GeNei) secondary antibody for 4 hrs. The blots were washed three times for 10 min each in 50mM TBS-T. Immunoreactivity was detected using DAB reagent (GeNei). As a control for equal loading, membranes were reprobed with mouse anti-β -actin antibody (Sigma).
Apoptosis
Apoptotic tumour cells were detected with TUNEL assay method, using the Apo-BrdU-IHC DNA fragmentation Assay Kit, Cat.K403-50 from BIOVISION. The assay was performed according to the manufacturer’s instruction. Briefly, after routine deparaffinization and treatment with 3% H2O2, sections were covered with 1X reaction buffer for 10 to 30 min and then with complete labelling reaction mixture (5X reaction buffer, Tdt enzyme, Br-dUTP, distilled water) for 1 to 1.5 hour. Sections were incubated with blocking buffer for 10 min and then with antibody solution (Anti-BrdU-biotin monoclonal antibody, blocking buffer) for 1 to 1.5 hour and then diluted conjugate solution (200X conjugate, Blocking buffer) was added to specimen for 30min. Slides were rinsed with PBS and incubated with diamino benzidine (DAB) chromogen solution in dark up to 15 min for colour development. Finally the sections were rinsed with distilled water and counter stained with methyl green followed by 2 dips of ethanol and mounted with DPX. Finally sections were observed under microscope and percentage of positively stained cells were calculated.
Statistical analysis
The statistical analysis was performed using SPSS 16 software. Intergroup differences were examined by using Chi-square test. Correlation analysis was done by Spearmann correlation. Survival analysis was done by Kaplan Meier method and Cox regression model was used for multivariate analysis of factors influencing the survival.
Power of study
With an alpha error of 5% and difference in caspase 3 expression of 24% between controls and breast cancer cases, the statistical power of study is 62%.
Results
Caspase 3 expression in breast cancer cases and controls
Caspase 3 protein expression by immunohistochemistry was seen in 69% of fibroadenoma controls (Figure 1 A,&B) and 48.9% of breast cancer cases (Figure1
C,&D). Caspase 3 protein expression by western blotting was expressed in 60% of controls and 36.7% of breast cancer cases. Higher caspase 3 expression was seen in controls compared to cases, the difference in expression was statistically significant (p<0.05). Caspase 3 mRNA was expressed in 38.9% of breast cancer cases and 62.1% of fibroadenoma controls. Significantly higher caspase 3 mRNA expression was seen in controls when compared to breast cancer (p+0.02). Immunohistochemical analysis of staining in breast cancer cases showed antigen expression in nucleus (11.4%(5/44)), cytoplasm [84.1%(37/44)] and both nuclear and cytoplasmic (4.5%(2/44)). In controls antigen expression was seen in nucleus [15%(3/20)] and cytoplasm (85%(17/20)). Weak (56.8%(25/44) cancer; 35%(7/20) control) to strong (13.6%(6/44) breast cancer; 15%(3/20) control) cytoplasmic caspase 3 expression was observed in both breast cancer cases and controls. In rest of the cases the expression was graded as moderate, the difference was statistically significant with controls showing relatively weak expression of caspase 3.
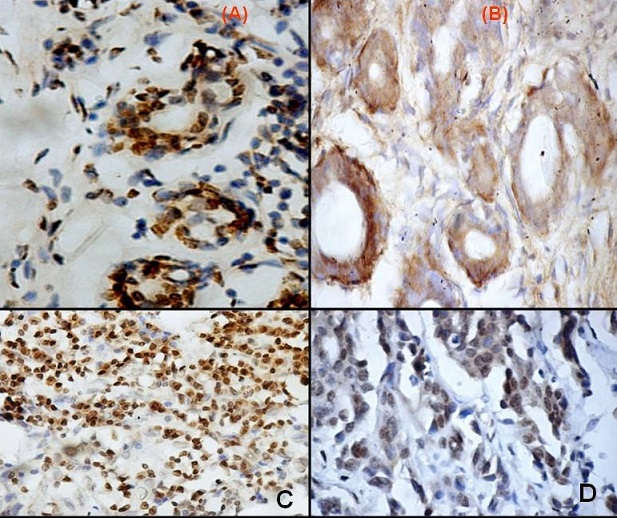

Figure 1: Photomicrograph showing immunohistochemistry for caspase 3 (A) Nuclear and (B) Cytoplasmic stain (x400) in fibroadenoma controls
(C) Nuclear(x100), (D) Nuclear & Cytoplasmic staining (x400)
Relationship of caspase 3 with socio-demographic and clinico-pathological parameters.
Caspase 3 mRNA expression was significantly related with breast cancer patients not habituated to chewing tobacco (p=0.013) while the protein expression showed significant association in breast cancer patients habituated to tobacco (p=0.038). Caspase 3 mRNA and protein expression did not show significant association with other socio-demographic variables (breast side, abortion, previous operation, religion and ethnic origin) in breast cancer cases.
Caspase 3 mRNA and protein expression showed significant association with tumour stage (p=0.002, p=0.009) and metastasis (p=0.001, p=0.003) in breast cancer cases (Table 1). Caspase 3 protein expression by immunohistochemistry also showed significant association with positive lymph node status in breast cancer cases (p=0.036) (Table 1). A single node negative breast cancer case also showed caspase 3 expression. Caspase 3 mRNA and protein expression did not show significant association with clinico-pathological variables (histological grade, oestrogen receptor, progesterone receptor and HER2 receptor status) in breast cancer cases (Table 1)
| Variable |
Caspase 3 Expression |
P value |
| |
Protein level(%) |
mRNA level(%) |
Protein level(%) |
mRNA level(%) |
| |
IHC |
Western blotting |
|
IHC |
Western blotting |
|
Stage
T
1
2
3
4
N
0
1
2
3
M
0
1
|
66.7
72.2
66.7
41.7
43.8
64.1
42.8
33.3
71.1
60
|
33.3
25
53.8
33.3
44.4
32
50
100
36.4
66.7
|
100
55.5
55.5
25
37.5
51.3
28.6
33.3
55.6
60
|
0.008** 0.13 0.000** |
0.009** 0.62
0.003**
|
0.002**
0.26
0.001**
|
Lymph Node Negative
Positive
|
100
55.1
|
0
36.6
|
0
44.9
|
0.03* |
0.22
|
0.09 |
Grade
1
2
3
4
|
100
53.5
51.7
100
|
0
42.3
33.3
100
|
0
48.8
34.5
100
|
0.18
|
0.29
|
0.13 |
Oestrogen Receptor
Negative
Positive
|
44.9
65.2
|
40
31.2
|
34.7
56.5
|
0.17 |
0.48 |
0.11 |
Progesterone Receptor Negative
Positive
|
44
71.4
|
40
31.2
|
36
57.1
|
0.05
|
0.20 |
0.11
|
HER2
Negative
Positive
|
42.8
56
|
33.3
37.5
|
42.8
42
|
0.29
|
0.38 |
0.44
|
Legend: T: Tumour stage, N: Node, M: Metastasis, *: statistically significant (p<0.05), ** statistically significant (p<0.01).
Apoptosis in breast cancer cases and fibroadenoma controls
Apoptosis was seen in 44.4% of breast cancer cases and 75.9% of controls. Apoptosis was seen to be higher and significant in fibroadenoma controls when compared to breast cancer cases (p=0.003). Weak [57.5%(23/40)] to moderate[30%/(12/40)] immunostaining intensity was seen in majority of breast cancer cases while in controls majority showed strong [54.5%(12/22)] and moderate [36.4%(8/22)] staining.
Relationship of apoptosis with socio-demographic and clinico-pathological parameters
Significantly higher apoptosis was seen with breast cancer patients having no metastasis (p=0.001) and patients habituated to tobacco (p=0.047) (Table 2). Apoptosis did not show significant association with other clinico-pathological variables (stage, histological grade, lymph node, oestrogen receptor, progesterone receptor and HER 2 receptor status) or with other socio-demographic variables (breast side, abortion, previous operation, religion and ethnic origin)in breast cancer cases (Table 2).
| Variable |
Apoptosis |
Chi square |
P Value |
Breast side
Left
Right
|
23/46(50%)
17/44(38.6%)
|
1.17 |
0.27
|
Menstrual
Status
Pre
Post
|
10/30(33.3%)
30/60(50%)
|
2.25 |
0.13
|
Abortion
Yes
No
|
7/20(35%)
33/70(47.1%)
|
0.92
|
0.33
|
Previous Operation
Yes
No
|
9/22(40.9%)
31/68(45.6%)
|
0.14
|
0.70
|
Ethnic Origin
Upper Caste
OBC
SC
|
22/47(46.8%)
14/33(42.4%)
4/10(40%)
|
0.24 |
0.88
|
Religion
Hindu
Muslim
|
35/76(46.1%)
5/14(35.7%)
|
0.51 |
0.47
|
Addiction
Yes
No
|
5/20(25%)
35/70(50%)
|
3.93 |
0.04* |
Stage
T
1
2
3
4
N
0
1
2
3
M
0
1
|
2/3(66.7%)
9/18(50%)
12/18(66.7%)
9/24(37.5%)
5/16(31.2%)
22/39(56.4%)
3/7(42.8%)
1/3(33.3%)
29/45(64.4%)
2/5(40%)
|
10.74
4.26
15.13
|
0.05*
0.37
0.001**
|
Lymph Node
Negative
Positive
|
0/1(0%)
35/69(50.7%)
|
4.96
|
0.08
|
Grade
1
2
3
4
|
0/1(0%)
21/43(48.8%)
13/29(44.8%)
1/1(100%)
|
3.51
|
0.47
|
Oestrogen Receptor
Negative
Positive
|
19/49(38.8%)
14/23(60.8%)
|
3.37
|
0.18
|
Progesterone Receptor
Negative
Positive
|
20/50(40%)
13/21(61.9%)
|
3.43
|
0.17
|
HER2
Negative
Positive
|
9/21(42.8%)
24/50(48%)
|
0.72
|
0.69
|
Legend: T: Tumour stage, N: Node, M: Metastasis, *: statistically significant (p<0.05), ** statistically significant (p<0.01). OBC: other backward caste, SC: scheduled caste, CI: Confidence interval, *: statistically significant (p<0.05), ** statistically significant (p<0.01).<h4>
Correlation between caspase 3 and apoptosis
Caspase 3 expression significantly and positively correlated with apoptosis by TUNEL assay method in breast cancer cases (r=0.69, p=0.000) and not with controls (r=0.31, p=0.09).
Survival analysis
Survival data of 70 breast cancer cases was available, of which 12.8% were dead and 87.1% were alive, rest of the patients were lost to follow-up. Overall 3-year Survival rate for patients with caspase 3-positive breast carcinomas (88.6%) was less than that for patients with caspase 3-negative tumours (91.3%), but the difference was statistically not significant (p=0.46) (Figure 2A). Similarly lowering of survival for apoptosis positive breast cancer (87.5%) compared to apoptosis negative cancer (92%) was observed however the difference was not statistically significant (p=0.10) (Figure 2B). Multivariate Cox Proportional Hazard model analysis demonstrated abortion (p=0.002), metastasis (p=0.002) and oestrogen receptor (p=0.04) status as significant independent predictor of survival in breast cancer cases. Caspase 3 expression and rate of apoptosis were not found to be significant predictor (Table 3).
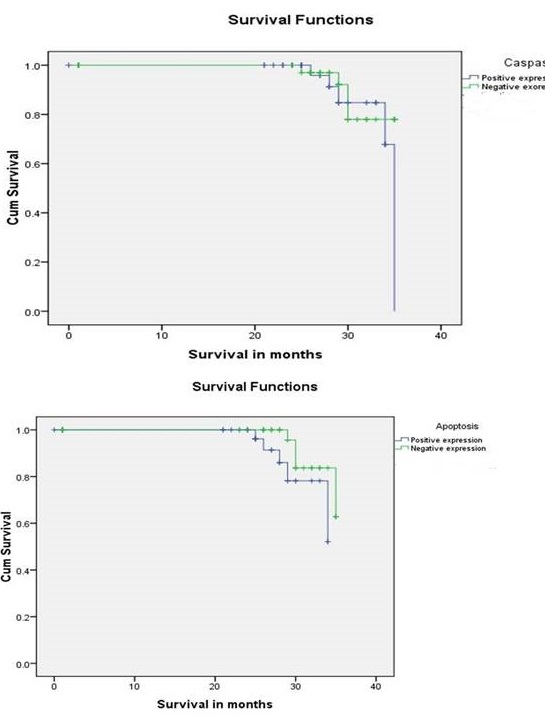

Figure 2: Kaplan-Meier curves showing A) overall survival in patients with breast carcinoma according to caspase 3 expression
B) overall survival in patients with breast carcinoma according to apoptosis by
TUNEL assay method.
| Characteristic |
β |
Significance |
Exp(β) |
95% Confidence Interval for Exp(β) |
| Menstrual status |
-0.87 |
0.12 |
0.41 |
0.13-1.26 |
| Parity |
0.08 |
0.53 |
0.91 |
0.70-1.19 |
| Abortion |
2.48 |
0.002** |
12.04 |
2.40-60.46 |
| T |
|
0.41 |
|
|
| T(1) |
-0.16 |
0.89 |
0.84 |
0.06-11.36 |
| T(2) |
1.37 |
0.15 |
3.96 |
0.60-25.98 |
| T(3) |
0.03 |
0.96 |
1.03 |
0.18-5.90 |
| T(4) |
0.24 |
0.78 |
1.28 |
0.21-7.54 |
| N |
|
0.57 |
|
|
| N(0) |
0.29 |
0.76 |
1.34 |
0.18-9.52 |
| N(1) |
-0.91 |
0.35 |
0.40 |
0.05-2.80 |
| N(2) |
-0.45 |
0.65 |
0.63 |
0.08-4.72 |
| N(3) |
-0.48 |
0.66 |
0.61 |
0.06-5.58 |
| M |
|
0.08 |
|
|
| M(0) |
1.27 |
0.02* |
3.56 |
1.15-11.00 |
| M(1) |
-1.38 |
0.07 |
3.25 |
0.05-1.13 |
| Grade |
|
0.45 |
|
|
| Grade(1) |
-3.51 |
0.17 |
0.03 |
0.00-4.82 |
| Grade(2) |
0.26 |
0.77 |
1.30 |
0.20-8.26 |
| Grade(3) |
0.59 |
0.54 |
1.81 |
0.26-12.32 |
| Grade(4) |
2.38 |
0.26 |
10.89 |
0.16-701.06 |
| Lymph node |
|
0.58 |
|
|
| Positive Lymph node |
-0.55 |
0.58 |
0.57 |
.08-4.10 |
| Oestrogen Receptor |
|
0.04* |
|
|
| Negative oestrogen receptor |
4.25 |
0.03* |
0.01 |
0.00-0.77 |
| Positive oestrogen Receptor |
-4.23 |
0.01* |
0.01 |
0.00-0.44 |
| progesterone receptor |
|
0.90 |
|
|
| Negative progesterone receptor |
0.09 |
0.90 |
1.09 |
0.24-4.98 |
| HER2 receptor |
|
0.66 |
|
|
| Negative HER2 receptor |
-0.19 |
0.66 |
0.82 |
0.34-1.97 |
| Survivin |
0.53 |
0.35 |
1.71 |
0.54-5.36 |
| XIAP |
-1.02 |
0.09 |
0.35 |
0.10-1.21 |
| Caspase 3 |
-0.67 |
0.38 |
0.50 |
0.11-2.31 |
| Apoptosis |
0.80 |
0.21 |
2.22 |
0.62-7.92 |
Discussion
Tumour growth is the net result of cell proliferation and cell loss by apoptosis. Caspase-3 (CPP32) expression has been considered to be directly correlated with apoptosis because of its location in the protease cascade pathway. Caspase-3 can be activated by diverse death-inducing signals, including the chemotherapeutic agents and radiation. Similar to the previous study [9], caspase 3 mRNA was highly expressed in controls when compared to breast cancer cases in the present study. results of caspase 3 protein expression in the present study were in contrast with those of an earlier report wherein an over expression was found to be involved with apoptosis in correlation with bcl2 and p53 pathway [11]. In our study, weak staining intensity in breast cancer cases and high to moderate in controls was seen suggesting down regulation of the caspase 3 in cancer, quantification of the expression could possibly be used as an biomarker for potential carcinogenesis. This is also in agreement with previous study [12], however in that study the expression of caspase did not correlate with the apoptosis as demonstrated by TUNEL assay, like it did in our study. The reason could be activation of apoptosis by pathways that work independent of caspase 3 and may have genetic or individual variations.
Caspase 3 in breast cancer has been shown to plays a critical role in radiotherapy and chemotherapy-induced apoptosis. It is suggested to be contributing to the radioresistance and chemoresistance in breast cancer cases [13-15]. Cell culture studies have demonstrated down regulation of caspase 3 by taxane treatment [16], thus suggesting a potential therapeutic role of caspase 3. Tumours with low levels of caspase has also been shown to have better response to 5FU based chemotherapy [17]. Caspase 3 expression in remnant tumour after neodajuvant therapy of breast cancer has been shown to be associated with poor prognosis [18]. All these results suggest better chemotherapeutic response in patients with low expression of caspase 3, and hence make caspase 3 an attractive therapeutic target that may help boost the response to chemotherapeutic agents including taxanes.
Mansoori et al., has shown significant decrease in expression of MiR-34a and let-7a in patients with breast cancer along with decrease in caspase 3, they have suggested that down regulation of miRNA could be a mechanism to evade apoptosis by cancer cells [19]. Similarly Over expression of miR-548-3p has been found to be associated with increased apoptosis and inhibition of cell growth [20]. A number of chemical molecules have been found to modulate caspase 3 expression in cell culture studies, paving the way for caspase 3 targeting [21, 22].
Caspase 3 mRNA and protein expression was significantly related with tumour stage and metastasis while caspase 3 protein also showed relation with lymph node status in agreement with previous results [12,23]. Earlier studies have failed to demonstrate any significant relation of caspase 3 with histological grade, oestrogen receptor, progesterone receptor and HER 2 receptor status in breast cancer [24] similar to the present study, where oestrogen receptor and lymph node status were found to be independent predictors of survival. Expression of apoptosis-related proteins has been found to predict lymph node metastasis and disease-free survival in breast cancer patients [25, however, in our study correlation and multivariate analysis failed to show any significant association between the two.
In contrast to previous results [24, 26], our results have shown higher and significant apoptosis in controls when compared to breast cancer cases. A significant relationship with breast cancer with lymph node metastasis was also observed in agreement with previous study [12]. Since we have found a relationship between apoptosis rate and caspase 3 in breast cancer cases we can say that execution of apoptosis is the most common function of caspase 3 and detection of caspase 3 is a reliable method for assessing apoptosis as this method is more sensitive, specific, is free of subjective interpretation and does not rely on DNA fragmentation which is a late event in apoptotic process.
Loss of caspase 3 expression in female breast cancer compared to controls have important clinical implications and caspase3 can be used not only as a marker to predict response to therapy, but also as a therapeutic target either directly or through other apoptosis related proteins like survivin to improve the response of chemotherapeutic agents. Treatment by survivin targeting short interfering RNA (siRNA) has been shown to reduce apoptosis in MCF-7 cells [27]. Similarly Rimcazole, a Sigma 1 Receptor antagonist has also been found to be effectively down regulating the apoptosis synergistically with p53 in tumour cells [28].In conclusion, our results show loss of caspase 3 expression in breast cancer compared to controls, these findings have important clinical implication and in conjunction with literature suggest that caspase 3 can be as a marker for predicting chemotherapetic response and also as a therapeutic target that may help in improving the response of adjuvant therapy.
Funding:
This work was supported in part by a grant-in-aid from Indian Council of Medical Research, New Delhi, India (grant no 5/13/120/2008 NCD-III).
Compliance with ethical standards
The study was approved by the Institute Ethics committee of Institute of Medical Sciences, Banaras Hindu University, Varanasi. Informed consent was obtained from each of the study participants.
Conflict of Interests
The authors declare that there are no conflict of interests
Authors' contribution
KJ: Conducted the experiments, collected the data and literature, and prepared the draft manuscript.
MS and MK: Conducted the histopatholgical examination and IHC of the specimens and wrote the pathological part of the manuscript.VKS: helped with design of the study and editing of the manuscript.
MP: conceived and designed the study, monitored the experiments and data collection and edited the final manuscript. MP is also guaranteer of the manuscript.
References
[1).ipponen PK, Aaltomaa S. Apoptosis in bladder cancer as related to standard prognostic factors and prognosis. J Pathol 1994 August;173(4):333-9.[PubMed]
[2].ustonen M, Raunio H, Paakko P, Soini Y. The extent of apoptosis is inversely associated with bcl-2 expression in premalignant and malignant breast lesions. Histopathology 1997 October;31(4):347-54.[PubMed]
[3]rardo MD, Elledge RM, de MC, Clark GM, Osborne CK, Allred DC. bcl-2 and apoptosis in lymph node positive breast carcinoma. Cancer 1998 April 1;82(7):1296-302.[PubMed]
[4].hen KL, Harn HJ, Ho LI, Yu CP, Chiu SC, Lee WH. The extent of proliferative and apoptotic activity in intraductal and invasive ductal breast carcinomas detected by Ki-67 labeling and terminal deoxynucleotidyl transferase-mediated digoxigenin-11-dUTP nick end labeling. Cancer 1998 June 15;82(12):2373-81.[PubMed]
[5].assan HI, Walker RA. Decreased apoptosis in non-involved tissue from cancer-containing breasts. J Pathol 1998 March;184(3):258-64.[PubMed]
[6].hornberry NA, Lazebnik Y. Caspases: enemies within. Science 1998 August 28;281(5381):1312-6.[PubMed]
[7].enault JB, Salvesen GS. Caspases: keys in the ignition of cell death. Chem Rev 2002 December;102(12):4489-500 [PubMed]
[8].iedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol 2004 November;5(11):897-907.[PubMed]
[9].evarajan E, Sahin AA, Chen JS et al. Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene 2002 December 12;21(57):8843-51.[PubMed]
[10] . Liu X, He Y, Li F et al. Caspase-3 promotes genetic instability and carcinogenesis. Mol Cell 2015 April 16;58(2):284-96.
[PubMed] [PMC
Full text]
[11] Nakopoulou L, Alexandrou P, Stefanaki K, Panayotopoulou E, Lazaris AC, Davaris PS. Immunohistochemical expression of caspase-3 as an adverse indicator of the clinical outcome in human breast cancer. Pathobiology 2001;69(5):266-73.[PubMed]
[12]. Grigoriev MY, Pozharissky KM,
Hanson KP, Imyanitov EN, Zhivotovsky B. Expression of caspase-3 and -7 does not
correlate with the extent of apoptosis in primary breast carcinomas. Cell Cycle
2002 September; 1(5): 337-42 .[PubMed]
[13]. Yang XH, Edgerton S, Thor AD. Reconstitution of caspase-3 sensitizes MCF-7 breast cancer cells to radiation therapy. Int J Oncol 2005 June; 26(6): 1675-80.[PubMed]
[14] .Yang XH, Sladek TL, Liu X, Butler BR, Froelich CJ, Thor AD. Reconstitution of caspase 3 sensitizes MCF-7 breast cancer cells to doxorubicin- and etoposide-induced apoptosis. Cancer Res 2001 January 1; 61(1): 348-54 [PubMed]
[Free Full
Text]
[15]. Blanc C, Deveraux QL, Krajewski S et al. Caspase-3 is essential for procaspase-9 processing and cisplatin-induced apoptosis of MCF-7 breast cancer cells. Cancer Res 2000 August 15;60(16):4386-90 [PubMed]
[Free Full
Text]
[16] Jelinek M, Balusikova K, Schmiedlova M et al. The role of individual caspases in cell death induction by taxanes in breast cancer cells. Cancer Cell Int 2015 February 4; 15(1): 8. [PubMed]
[PMC Full text]
[17] Flanagan L, Meyer M, Fay J et al. Low levels of Caspase-3 predict favourable response to 5FU-based chemotherapy in advanced colorectal cancer: Caspase-3 inhibition as a therapeutic approach. Cell Death Dis 2016 February 4;
7: e2087. [PubMed]
[PMC Full Text]
[18] Himuro T, Horimoto Y, Arakawa A et al. Activated Caspase 3 Expression in Remnant Disease After Neoadjuvant Chemotherapy May Predict Outcomes of Breast Cancer Patients. Ann Surg Oncol 2016 July; 23(7): 2235-41 [PubMed]
[19] Mansoori B, Mohammadi A, Shirjang S, Baghbani E, Baradaran B. Micro RNA 34a and Let-7a Expression in Human Breast Cancers is Associated with Apoptotic Expression Genes. Asian Pac J Cancer Prev 2016;17(4):1887-90.[PubMed]
[Free
Full Text]
[20] Shi Y, Qiu M, Wu Y, Hai L. MiR-548-3p functions as an anti-oncogenic regulator in breast cancer. Biomed Pharmacother 2015 October;75:111-6.
[PubMed]
[21] Zhao Y, Jing Z, Li Y, Mao W. Berberine in combination with cisplatin suppresses breast cancer cell growth through induction of DNA breaks and caspase-3-dependent apoptosis. Oncol Rep 2016 July; 36(1): 567-72. [PubMed]
[Free
Full Text]
[22] Shah P, Djisam R, Damulira H, Aganze A, Danquah M. Embelin inhibits proliferation, induces apoptosis and alters gene expression profiles in breast cancer cells. Pharmacol Rep 2016 June;68(3):638-44.[PubMed]
[23] Nassar A, Lawson D, Cotsonis G, Cohen C. Survivin and caspase-3 expression in breast cancer: correlation with prognostic parameters, proliferation, angiogenesis, and outcome. Appl Immunohistochem Mol Morphol 2008 March;16(2):113-20.[PubMed]
[24] Vakkala M, Paakko P, Soini Y. Expression of caspases 3, 6 and 8 is increased in parallel with apoptosis and histological aggressiveness of the breast lesion. Br J Cancer 1999 October;81(4):592-9.[PubMed]
[25] Kim H, Lee KH, Park IA et al. Expression of SIRT1 and apoptosis-related proteins is predictive for lymph node metastasis and disease-free survival in luminal A breast cancer. Virchows Arch 2015 November;467(5):563-70.[PubMed]
[26] O'Donovan N, Crown J, Stunell H et al. Caspase 3 in breast cancer. Clin Cancer Res 2003 February;9(2):738-42.[PubMed]
[27] Li Y, Li Y, Lee RJ et al. Antitumor activity of a novel survivin siRNA. Pak J Pharm Sci 2015 September;28(5 Suppl):1887-90.[PubMed]
[28] Happy M, Dejoie J, Zajac CK et al. Sigma 1 Receptor antagonist potentiates the anti-cancer effect of p53 by regulating ER stress, ROS production, Bax levels, and caspase-3 activation. Biochem Biophys Res Commun 2015 January 9;456(2):683-8.[PubMed]


