Research

Expression of X-linked inhibitor of apoptosis protein (XIAP) correlates with the lymph node and receptor status in breast cancer and reduces survival.
*Manoj Pandey,
*Kumkum Jha,
#Mohan Kumar,
#Mridula Shukla, $Vijay K Shukla,
- *Departments of Surgical Oncology, #Pathology and
$General Surgery Institute of Medical Sciences, Banaras Hindu University, Varanasi 221 005, India
- Submitted: September 14, 2016
- Accepted: October 30, 2016
- Published: November 4, 2016
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Background
X-linked inhibitor of apoptosis protein (XIAP), also known as MIHA/hILP/BIRC4 is a novel member of the inhibitors of apoptosis (IAP) family located on chromosome Xq25. XIAP has the most potent anti-apoptotic effect in cells and, is able to inhibit the effector caspases -3,-7 and -9. This case control study reports the mRNA and protein expression of XIAP, apoptosis and its effect on the survival in breast cancer.
Materials and Methods
XIAP expression was measured by immunohistochemistry, western Blotting and RT PCR method in 29 controls and 90 patients with breast cancer. Apoptosis was detected by using based on in situ labelling of the fragmented DNA (terminal deoxynucleotidyl transferase mediated UTP nick end-label (TUNEL)) staining.
Results
XIAP protein expression was seen in 62.1% of controls and 86.7% of breast cancers, while mRNA expression was seen in 51.7% of controls and 87.8% of breast cancer. The differences were statistically significant. past history of abortion (p=0.002), metastasis (p=0.002) and oestrogen receptor (p=0.04) were found as independent and significant predictor for overall survival in breast cancer. XIAP negative patients had higher survival; however, the difference was not statistically significant.
Conclusion
XIAP is an important biomarker of breast cancer. Its expression significantly correlates with expression of hormone receptors, and prognosis is better in XIAP negative breast cancer.
Introduction
Breast cancer is the most common malignancy and the leading cause of morbidity and mortality in women. Breast development is controlled by a balance between cell proliferation and apoptosis. Tumour cells acquire resistance to apoptosis by expression of certain anti – apoptotic proteins called Inhibitors of Apoptosis proteins (IAP). cIAP1, cIAP2, NAIP, Survivin, XIAP, apollon, ILP-2 and livin are the 8 IAPs (IAP) that has been identified in humans. XIAP (X linked inhibitor of apoptosis protein) was first isolated in 1997, also known as MIHA/hILP/BIRC4 a potent endogenous inhibitor, is the prototype of the IAP family. XIAP a 55 kDa, cytoplasmic protein, located on chromosome Xq25 [1,2], has unique structure with three BIRs and a typical zinc binding domain [3]. XIAP has the most potent anti-apoptotic effect in cells and is able to inhibit the effector caspases -3,-7 and -9 [4-6]. Increased expression of XIAP has been shown to be associated with aggressive malignant behaviour and disease progression in lymphoma, breast cancer, lung cancer and renal cell carcinoma [7-9]. Over expression of XIAP has been correlated with poor prognosis for some types of cancer, while converse where either XIAP is not prognostic or high XIAP levels correspond to a longer survival have also been reported [10-13] . XIAP mRNA is observed in all adult and foetal tissues except peripheral blood leukocytes, indicating that it is ubiquitously expressed member of the family [14].
In the present study expression of XIAP protein estimated by immunohistochemistry and western blotting, and mRNA expression was studied by RT PCR. Terminal TdT-mediated dUTP nick-end labelling (TUNEL) was performed to detect apoptosis in present study. Relationship among expression status of XIAP, apoptosis, clinico-pathological parameters, socio-demographic parameters and prognosis was studied.
Materials and Methods
Patients
After obtaining a written informed consent samples were obtained from 90 patients of breast cancer and 29 benign breast diseases (fibroadenoma) attending the Surgical Oncology and General Surgery outpatient, and scheduled to undergo surgery as part of their treatment. The study was approved by the institute ethics committee. After collection, the samples were stored in RNA latter for mRNA study and also snapped freeze in liquid nitrogen for protein study and were stored at -84 °C till further processing. A portion of the sample was stored in buffered formalin for histology and immunohistochemistry.
Immunohistochemistry
Four micron sections were prepared on poly L-lysine coated glass slides from the formalin fixed paraffin embedded tissues. Sections were transferred to xylene with three changes of 5 min each. Rehydration was done with graded alcohol from 100% to 70% followed by running tap water for 30 min. Antigen retrieval was done in citrate buffer for 20 min. Slides were washed 3 times after retrieval in TBS for 10 minutes each. Endogenous peroxidise activity was blocked by incubating section with 0.3% peroxide (H202) in methanol for 20 minutes. Sections were washed in TBS for 5 minutes and were incubated in normal blocking serum for 1 hour to block non-specific binding sites. Slides were incubated overnight with XIAP antibody (XIAP (H-202): sc-11426, Santa Cruz Biotechnology, Inc.). Slides were washed in TBS for 5 minutes. Secondary staining was done as per manufacturer’s instruction using Vectastain Universal Elite Abc Kit from Vector laboratories (Cat.No.PK-6200). Slides were washed for 5 minutes in TBS. Sections were incubated in peroxidise substrate solution for colour development. Finally sections were counterstained with haematoxylin for observation under light microscope for image analysis and quantification.
Grading of immunostaining
Immunostaining intensity was graded as Strong (+++), Moderate
(++), Weak (+) or nil (0) staining.
RT-PCR
Total RNA was extracted by TRIZOL (Invitrogen) and reverse transcribed to cDNA with High Capacity cDNA Reverse Transcription Kits (Applied Biosystem). PCR reactions were performed using GAPDH as an internal standard. Primers used were: XIAP forward: 5’- CAACACTGGCACGAGCAGGG-3’, XIAP Reverse 5’-CATGGCAGGGTTCCTCGGGT-3’. The predicted length of the amplified product was 347bp [15]; GAPDH forward primer (FP): 5’-ACGGATTTGGTCGTATTGGGCG-3’, GAPDH reverse primer (RP) 5’-CTCCTGGAAGATGGTGATGG-3’. The predicted length of that amplified product was 212 bp [16]. The PCR cycles were set for total 33 cycles with denaturation temperature at 94 ℃ for 30 seconds, annealing temperature at 54 ℃ for 40 seconds and extending temperature at 72 ℃ for 1 min per cycle and last cycle lasted for 10 min at 72 ℃.
Western Blotting
Tissue were diluted in sodium dodecyl sulphate (SDS) buffer containing 50mM Tris-HCL (pH 6.8), 4% beta mercaptoethanol, 4% SDS, 20% glycerol and 0.01% bromophenol blue. Protein from each sample was separated on a 12% SDS–polyacrylamide gel. Following electrophoresis, the separated proteins were transferred to a PVDF transfer membrane (Thermoscientific) using a wet blotting apparatus for overnight. The membranes were then treated for one hour at room temperature with a blocking solution containing 50mM Tris buffered saline (TBS), 5% skimmed milk powder and 0.05% Triton-X-100 (TBS-T).Following this, the membranes were incubated overnight at 4ºC with XIAP antibody (XIAP (H-202): sc-11426, Santa Cruz Biotechnology, Inc.), in a blocking solution containing 50mM TBS-T and 5% skimmed milk powder. Subsequently, membranes were washed in 50mM TBS containing 0.05% Triton and then probed with Goat anti mouse IgG-HRP (GeNei) secondary antibody for 4 hours. The blots were washed three times for 10 min each in 50mM TBS-T. Immunoreactivity was detected using DAB reagent (GeNei). As a control for equal loading, membranes were reprobed with mouse anti-β -actin antibody (Sigma).
Apoptosis
Apoptotic tumour cells were detected with TUNEL assay method, using the Apo-BrdU-IHC DNA fragmentation Assay Kit, Cat.K403-50 from BIOVISION. The assay was performed according to the manufacturer’s instruction. Briefly, after routine deparaffinization and treatment with 3% H2O2, sections were covered with 1X reaction buffer for 10 to 30 min and then with complete labelling reaction mixture (5X reaction buffer, Tdt enzyme, Br-dUTP, distilled water) for 1 to 1.5 hour. Sections were incubated with blocking buffer for 10 min and then with antibody solution (Anti-BrdU-biotin monoclonal antibody, blocking buffer) for 1 to 1.5 hour and then diluted conjugate solution (200X conjugate, Blocking buffer) was added to specimen for 30min. Slides were rinsed with PBS and incubated with diamino benzidine (DAB) chromogen solution in dark up to 15 min for colour development. Finally the sections were rinsed with distilled water and counter stained with methyl green followed by 2 dips of ethanol and mounted with DPX. Finally sections were observed under microscope and percentage of positively stained cells to total cells were calculated.
Statistical analysis
The statistical analysis was performed using SPSS 16 software. Intergroup differences were examined by using Chi-square test. Correlation analysis was done by Spearmann correlation. Survival analysis was done by Kaplan Meier method and Cox regression hazard model.
Results
XIAP expression in breast cancer cases and fibroadenoma controls
XIAP expression by immunohistochemistry was seen in 86.7% of breast cancer 0 (Figure 1A ), and 62.1% of controls (p=0.0040 Figure 1B ). XIAP mRNA was expressed in 51.7% of controls (p=0.0000Figure 1C ,) and 87.8% of breast cancer cases Figure 1C ,). XIAP protein expression by western blotting was observed in 52.4 % of controls (p=0.002 Figure 1D ,)) and 86.1% of breast cancer cases. Significantly higher XIAP mRNA (p=0.00) and protein (p=0.04) expression was seen in breast cancer cases when compared to controls (Table 1). Immunohistochemical analysis showed weak to strong XIAP cytoplasmic expression in both cases and controls.
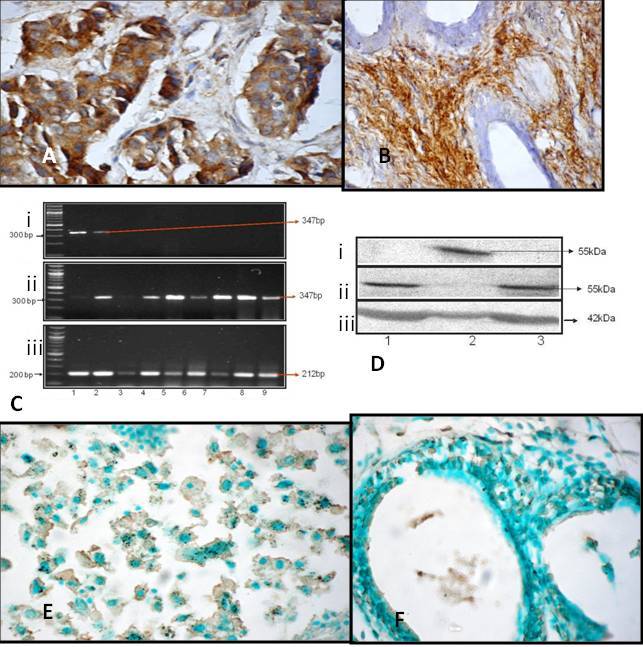
Figure 1: XIAP expression in breast cancer and controls. A). Photomicrograph
showing moderate immunohitochemical staining for XIAP in carcinoma breast
(X400). B) Photomicrograph showing moderate immunohitochemical staining for XIAP
in controls. C) XIAP expression in (i) Fibroadenoma controls (ii) Breast Cancer
cases (iii) GAPDH as control; D) XIAP expression in (i) Fibroadenoma controls
(ii) Breast cancer cases (iii) beta actin as control; E) Strong apoptosis stain
in breast cancer cases F) Apoptotic stain in fibroadenoma controls
Relationship of XIAP with socio-demographic and clinico-pathological parameters.
XIAP mRNA and protein expression did not show significant association with socio-demographic variables (breast side, abortion, previous operation, religion and ethnic origin) in breast cancer cases (Table 1) and other clinico-pathological variables (stage, histological grade and HER2 receptor status), except significantly higher mRNA expression in breast cancer cases that were not habituated to tobacco (p=0.04). XIAP protein (p=0.044, 0.049 respectively) and mRNA (p=0.022, 0.04 respectively) expression showed significant association with positive lymph node and positive progesterone receptor status in breast cancer cases (Table 1). XIAP protein expression by western blotting showed significant association with positive oestrogen (p=0.025) and positive progesterone receptor status (p=0.019) (Table 1). XIAP expression at mRNA and protein level did not show significant association with in breast cancer cases (Table 1).
|
Variable
|
XIAP Expression
|
Chi square
|
P
value
|
|
|
Protein
|
mRNA
|
Protein
|
mRNA
|
Protein
|
mRNA
|
|
Breast
side
Left
Right
|
38/46(82.6%)
40/44(90.9%)
|
38/46(82.6%)
41/44(93.2%)
|
1.34
|
2.34
|
0.24
|
0.12
|
|
Menstrual
Status
Pre
Post
|
26/30(86.7%)
52/60(86.7%)
|
27/30(90%)
52/60(86.7%)
|
0.00
|
0.20
|
1.00
|
0.64
|
|
Abortion
Yes
No
|
17/20(85%)
61/70(87.1%)
|
17/20(85%)
62/70(88.6%)
|
0.06
|
0.18
|
0.80
|
0.66
|
|
Previous
Operation
Yes
No
|
20/22(90.9%)
58/68(85.3%)
|
20/22(90.9%)
59/68(86.8%)
|
0.45
|
0.26
|
0.50
|
0.60
|
|
Ethnic
Origin
Upper
Caste
OBC
SC
|
41/47(87.2%)
29/33(87.9%)
8/10(80%)
|
41/47(87.2%)
30/33(90.9%)
8/10(80%)
|
0.44
|
0.87
|
0.80
|
0.64
|
|
Religion
Hindu
Muslim
|
65/76(85.5%)
13/14(92.9%)
|
66/76(86.8%)
13/14(92.9%)
|
0.55
|
0.39
|
0.45
|
0.52
|
|
Addiction
Yes
No
|
15/20(75%)
63/70(90%)
|
15/20(75%)
64/70(91.4%)
|
3.02
|
3.91
|
0.08
|
0.04*
|
|
T Stage
T1
T2
T3
T4
Tx
|
3/3
(100%)
16/18
16/18
21/24
22/27
|
3//3
16/18
16/18
22/24
22/27
|
1.55
|
1.93
|
0.9
|
0.85
|
|
N stage
N0
N1
N2
N3
Nx
|
13/16
35/39
7/7
3/3
21/25
|
14/16
34/39
7/7
3/3
21/25
|
2.10
|
1.73
|
0.71
|
0.78
|
|
M stage
M0
M1
Mx
|
42/45
4/5
32/40
|
42/45
4/5
33/40
|
3.46
|
2.61
|
0.17
|
0.27
|
|
Lymph
node
Negative
Positive
unknown
|
13/16
45/49
21/25
|
14/16
44/49
21/25
|
6.24
|
7.62
|
0.04*
|
0.02*
|
|
Grade
I
II
III
X
|
1/1
39/43
27/30
11/16
|
1/1
40/43
27/30
11/16
|
5.58
|
6.87
|
0.23
|
0.14
|
|
ER
Positive
Negative
|
22/23
43/49
|
22/23
44/49
|
4.9
|
5.57
|
0.08
|
0.06
|
|
PR
Positive
Negative
|
21/21
43/50
|
21/21
44/50
|
6.02
|
6.44
|
0.04*
|
0.04
|
|
HER 2
Positive
Negative
|
46/50
18/21
|
47/50
18/21
|
4.01
|
5.4
|
0.13
|
0.06
|
Legend: OBC: other backward caste, SC: scheduled caste, CI: Confidence interval;*: statistically significant (p<0.05), T: Tumour stage, N: Node, M: Metastasis, IHC –immunohistochemistry, WB-Western blot. ER Estrogen receptor, PR- progestron receptor
Power of Study
With an alpha error of 5% and difference in XIAP expression of 36% between controls and breast cancer cases, the statistical power of this study is 97%.
Expression of apoptosis in breast cancer cases and controls
Apoptosis was observed in 44.4% of breast cancer cases (Figure 1E ) and 75.9% of controls (Figure 1F )). Apoptosis was higher and significant in controls compared to breast cancer (p=0.003). In breast cancer 57.5% showed weak immunostaining intensity where as in controls 54.5% showed strong intensity.
Relationship of apoptosis with socio-demographic and clinico-pathological parameters
Apoptosis did not show significant association with socio-demographic variables (breast side, abortion, previous operation, religion and ethnic origin) in breast cancer cases and other clinico-pathological variables (stage, histological grade, lymph node, oestrogen receptor, progesterone receptor and HER 2 receptor status), except significantly higher apoptosis in breast cancer patients not habituated to tobacco (p=0.047). Significantly higher apoptosis was also observed in patients not having metastatic disease (p=0.001) (Table 2). XIAP expression (r=0.12, p=0.16) did not correlate significantly with apoptosis.
|
Sociodemographic Variable
|
Apoptosis
|
Chi square
|
P Value
|
|
Breast side
Left
Right
|
23/46(50%)
17/44(38.6%)
|
1.17
|
0.27
|
|
Menstrual
Status
Pre
Post
|
10/30(33.3%)
30/60(50%)
|
2.25
|
0.13
|
|
Abortion
Yes
No
|
7/20(35%)
33/70(47.1%)
|
0.92
|
0.33
|
|
Previous Operation
Yes
No
|
9/22(40.9%)
31/68(45.6%)
|
0.14
|
0.70
|
|
Ethnic Origin
Upper Caste
OBC
SC
|
22/47(46.8%)
14/33(42.4%)
4/10(40%)
|
0.24
|
0.88
|
|
Religion
Hindu
Muslim
|
35/76(46.1%)
5/14(35.7%)
|
0.51
|
0.47
|
|
Addiction
Yes
No
|
5/20(25%)
35/70(50%)
|
3.93
|
0.04*
|
|
Clinicopatholgical
variables
|
|
|
|
|
Stage
T
1
2
3
4
N
0
1
2
3
M
0
1
|
2/3(66.7%)
9/18(50%)
12/18(66.7%)
9/24(37.5%)
5/16(31.2%)
22/39(56.4%)
3/7(42.8%)
1/3(33.3%)
29/45(64.4%)
2/5(40%)
|
10.74
4.26
15.13
|
0.05
0.37
0.001**
|
|
Lymph Node
Negative
Positive
|
0/1(0%)
35/69(50.7%)
|
4.96
|
0.08
|
|
Grade
1
2
3
4
|
0/1(0%)
21/43(48.8%)
13/29(44.8%)
1/1(100%)
|
3.51
|
0.47
|
|
Oestrogen Receptor
Negative
Positive
|
19/49(38.8%)
14/23(60.8%)
|
3.37
|
0.18
|
|
Progesterone Receptor
Negative
Positive
|
20/50(40%)
13/21(61.9%)
|
3.43
|
0.17
|
|
HER2
Negative
Positive
|
9/21(42.8%)
24/50(48%)
|
0.72
|
0.69
|
Legend: Legend: OBC: other backward caste, SC: scheduled caste, CI: Confidence interval, *: statistically significant (p<0.05), ** statistically significant (p<0.01) T: Tumour stage, N: Node, M: Metastasis, *: statistically significant (p<0.05), ** statistically
Survival analysis
Survival data was available for 70 breast cancer cases, out of which 12.8% were dead and 87.1% were alive at the end of three year follow-up. XIAP expression correlated significantly with survival in breast cancer cases (r=0.20, p=0.04), whereas apoptosis did not correlate with survival.
Overall 3-year Kaplan-Meier survival rate for patients with XIAP-positive breast carcinomas (88.5%) was less than that for patients with XIAP-negative tumours (100%), but the difference was not significant (p=0.411)(Figure 2A ). Survival rates were lower for patients with apoptosis (87.5%) compared to apoptosis negative cases (92%) however, the difference was not statistically significant (p=0.100) (Figure 2B ). Multivariate Cox Proportional Hazard model demonstrated abortion (p=0.002), metastasis (p=0.002) and oestrogen receptor (p=0.04) as independent and significant predictor for overall survival in breast cancer cases. XIAP and apoptosis did not turn out to be a significant independent predictor for overall survival in breast cancer (Table 3).
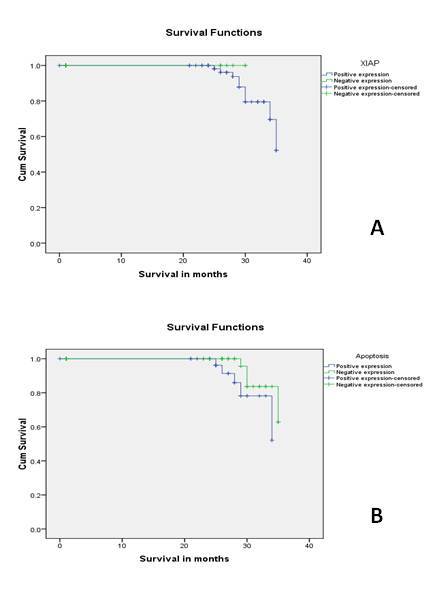
Figure 2: Survival A) Kaplan-Meier curves for overall survival rates of
patients with breast carcinoma categorized according to XIAP expression B)
Kaplan-Meier curves for overall survival rates of patients with breast carcinoma
categorized according to apoptosis by TUNEL assay
|
Characteristic
|
β
|
Significance
|
Exp(β)
|
95%
Confidence Interval for Exp(β)
|
|
Menstrual status
|
-0.87
|
0.12
|
0.41
|
0.13-1.26
|
|
Parity
|
-0.08
|
0.53
|
0.91
|
0.70-1.19
|
|
Abortion
|
2.48
|
0.002**
|
12.04
|
2.40-60.46
|
|
T
|
|
0.41
|
|
|
|
T(1)
|
-0.16
|
0.89
|
0.84
|
0.06-11.36
|
|
T(2)
|
1.37
|
0.15
|
3.96
|
0.60-25.98
|
|
T(3)
|
0.03
|
0.96
|
1.03
|
0.18-5.90
|
|
T(4)
|
0.24
|
0.78
|
1.28
|
0.21-7.54
|
|
N
|
|
0.57
|
|
|
|
N(0)
|
0.29
|
0.76
|
1.34
|
0.18-9.52
|
|
N(1)
|
-0.91
|
0.35
|
0.40
|
0.05-2.80
|
|
N(2)
|
-0.45
|
0.65
|
0.63
|
0.08-4.72
|
|
N(3)
|
-0.48
|
0.66
|
0.61
|
0.06-5.58
|
|
M
|
|
0.08
|
|
|
|
M(0)
|
1.27
|
0.02*
|
3.56
|
1.15-11.00
|
|
M(1)
|
-1.38
|
0.07
|
0.25
|
0.05-1.13
|
|
Grade
|
|
0.45
|
|
|
|
Grade(1)
|
-3.51
|
0.17
|
0.03
|
0.00-4.82
|
|
Grade(2)
|
0.26
|
0.77
|
1.30
|
0.20-8.26
|
|
Grade(3)
|
0.59
|
0.54
|
1.81
|
0.26-12.32
|
|
Grade(4)
|
2.38
|
0.26
|
10.89
|
0.16-701.06
|
|
Lymph node
|
|
0.58
|
|
|
|
Positive Lymph node
|
-0.55
|
0.58
|
0.57
|
.08-4.10
|
|
Oestrogen Receptor
|
|
0.04*
|
|
|
|
Negative oestrogen receptor
|
-4.25
|
0.03*
|
0.01
|
0.00-0.77
|
|
Positive oestrogen Receptor
|
-4.23
|
0.01*
|
0.01
|
0.00-0.44
|
|
progesterone receptor
|
|
0.90
|
|
|
|
Negative progesterone
receptor
|
0.09
|
0.90
|
1.09
|
0.24-4.98
|
|
HER2 receptor
|
|
0.66
|
|
|
|
Negative HER2 receptor
|
-0.19
|
0.66
|
0.82
|
0.34-1.97
|
|
Survivin
|
0.53
|
0.35
|
1.71
|
0.54-5.36
|
|
XIAP
|
-1.02
|
0.09
|
0.35
|
0.10-1.21
|
|
Caspase 3
|
-0.67
|
0.38
|
0.50
|
0.11-2.31
|
|
Apoptosis
|
0.80
|
0.21
|
2.22
|
0.62-7.92
|
Legend: T: Tumour stage, N: Node, M: Metastasis, *: statistically significant (p<0.05), ** statistically significant (p<<0.01).
Discussion
XIAP has emerged as one of the most powerful inhibitors of apoptosis, due to its ability to block both the extrinsic and intrinsic pathways. Increased expression of XIAP has been found in various tumours and over expression of this protein was correlated with patients’ sensitivity to anticancer drugs and prognosis [17]. Researchers have also found that XIAP has a dominant contribution to breast cancer progression and chemoresistance [18]. Recent observations also suggest an important role for XIAP as a key regulator of tumour cell susceptibility to anti cancer drug [8, 19
-23].
Our results of XIAP mRNA expression showed it to be highly expressed in breast cancer cases when compared to controls. XIAP protein expression by immunohistochemistry was almost similar to earlier report [24]. As XIAP expression is known to be also post transcriptionally regulated so western blot analysis was also performed which showed results in agreement with immunohistochemistry. A similar correlation between XIAP mRNA and protein was previously reported in non–small-cell lung carcinomas [25]. Immunohistochemical analysis study showed that XIAP has a cytoplasmic expression in agreement with previous studies [26, 27]. This cytoplasmic protein plays a vital role in breast cancer cases by interacting with specific caspases, which are required for the cleavage of certain proteins involved in the disassembly of cell during apoptosis. Xu et al, 2014 studied the XIAP expression by tumor microarray and found higher expression of XIAP-C in breast cancer patients. In their study this correlated with expression of HER2 neu and they proposed that it may alos have prognostic importance [28].
In our study XIAP mRNA and protein expression did not show significant relationship with socio-demographic parameters like breast side, menstrual status, ethnic origin, religion and addiction in breast cancer cases, however XIAP mRNA expression showed significant relationship with breast cancer cases not habituated to chewing tobacco. XIAP mRNA and protein expression did not correlate significantly with menstrual status [26] and first pregnancy in breast cancer cases.
In contrast to previous study [26], our study has shown XIAP mRNA and protein expression to be significantly related with lymph node metastasis and positive progesterone receptor status. Our results are also in agreement with previous reports [26] that have shown no significant relationship of XIAP with tumour stage, histological grading, oestrogen receptor, and HER 2 receptor status in breast cancer cases.
In contrast to previous studies [29, 30], our results show higher and significant apoptosis in controls when compared to breast cancer cases. Apoptosis staining intensity was weak in breast cancer cases when compared to strong staining intensity in controls, suggesting inhibition of apoptosis in breast cancer.
In agreement with previous study [29], no significant relationship with clinico-pathological parameters like tumour stage, histological grade, lymph node status, oestrogen receptor, progesterone receptor and HER2 receptor status was observed with apoptosis. Similarly we too failed to find any association of XIAP with apoptosis [24, 26].Spearman’s rho correlation results are in agreement with previous study in non small cell lung cancer patients [11] that showed XIAP to be significantly and positively correlated with survival in breast cancer cases. This possible association of XIAP with survival was unexpected based on in vitro data indicate an anti apoptotic role for XIAP. Kaplan-Meier survival analysis showed overall 3 year survival rate for patients with XIAP-positive breast carcinomas was less than that for patients with XIAP negative tumours, but the difference was not significant, in agreement with previous study [30]. This observation has to be studied in detail with larger sample size. XIAP expression has also been found to be regulated via miR 27a [31], miR 429 [ 32] and miR 200c [33], exact significance of these is still to be understood.
In conclusion, our study has shown significantly higher XIAP expression in breast cancer coupled with higher power of the study we can conclude that XIAP can be used as a novel biomarker in breast cancer. The conflicts between mRNA and protein expression suggest post transcriptional regulation and this need to be verified in further studies. The expression of this biomarker need to be studied further in larger patient cohorts to examine their role as predictive and prognostic marker for long-term treatment outcome.
Funding
This work was supported in part by a grant-in-aid from Indian Council of Medical Research, New Delhi, India (grant no 5/13/120/2008 NCD-III).
Compliance with ethical standards
The study was approved by the Institute Ethics committee of Institute of Medical Sciences, Banaras Hindu University, Varanasi. Informed consent was obtained from each of the study participants.
Conflict of Interests
The authors declare that there are no conflict of interests.
References
[1]Separovic RE, Liston P, Lefebvre C, Korneluk RG. Assignment of human inhibitor of apoptosis protein(IAP) genes xiap, hiap-1, and hiap-2 to chromosomes Xq25 and 11q22-q23 by fluorescence in situ hybridization. Genomics 1996;37:404-6[PubMed]
[2]Stehlik C, Martin DR, Kumabashiri I, Schmid JA, Binder BR, Lipp J. Nuclear factor (NF)-nB-regulated X-chromosome-linked IAP gene expression protects endothelial cells from tumor necrosis factor a-induced apoptosis. J Exp Med 1998; 188:211.
[Pubmed]
[Full text]
[3].Choi YJ, Kim TG, Kim YH, Lee SH, Kwon YK, Suh SI, et al., Immunosuppressant PG490 (triptolide) induces apoptosis through the activation of caspase-3 and down-regulationof XIAP in U937 cells. Biochem Pharmacol 2003;66: 273–280. [PubMed]
[4]. Deveraux QL, Leo E, Stennicke HR. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J 1999; 18:5242-5250 [PubMed]
[5]Huang Y, Park YC, Rich RL. Structural basis of caspase inhibition by XIAP: differential roles of the linker versus the BIR domain. Cell 2001; 104: 781-790.[PubMed]
[6]Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci U S A 2001; 98: 8662-8667.
[Pubmed]
[Full text]
[7Muris JJ, Cillessen SA, Vos W, Houdt VIS, Kummer JA, Krieken VJH, et al., Immunohistochemical profiling of caspase signaling pathways predicts clinical response to chemotherapy in primary nodal diffuse large B-cell lymphomas. Blood 2005; 105(7):2916-2923.
[Pubmed]
[Full
text]
[8].Parton M, Krajewski S, Smith I, Krajewska M, Archer C, Naito M, et al., Coordinate expression of apoptosis-associated proteins in human breast cancer before and during chemotherapy. Clin Cancer Res 2002; 8(7):2100-2108.
[Pubmed]
[Full text]
[9].Ramp U, Krieg T, Caliskan E, Mahotka C, Ebert T, Willers R ,et al., XIAP expression is an independent prognostic marker in clear-cell renal carcinomas. Hum Pathol 2004; 35(8):1022-1028 [PubMed]
[10].Carter BZ, Kornblau SM, Tsao T, Wang RY, Schober WD, Milella M, et al., Caspase –independent cell death in AML: caspase inhibition in vivo with pan-caspase inhibitors or in vivo by XIAP or surviving does not affect cell survival or prognosis. Blood 2003; 102, 4179-4186.
[Pubmed]
[Full
text]
[11].. Ferreira CG, Valk VP, Span SW, Ludwig I, Smit EF, Kruyt FA,et al., Expression of x-linked inhibitor of apoptosis as a novel prognostic marker in radically resected non-small cell cancer patients. Clin.Cancer Res.2001; 7, 2468-2474[PubMed]
[12].Tamm I, Richter S, Scholz F, Schmelz K, Oltersdorf D, Karawajew L, et al., XIAP expression correlates with monocytic differentiation in aduct de novo AML: impact on prognosis. Hematol J.2004;89,489-495 [Pubmed]
[13]. Tamm I, Richter S, Oltersdorf D, Creutzig U, Harbott J, Scholz F, et al., High expression levels of X-linked inhibitor of apoptosis protein and surviving correlate with poor overall survivial in childhood de novo acute myeloid leukaemia. Clin.Cancer Res.2004; 102,
3737-3744. [Pubmed]
[Full
text]
[14]Liston PN, Roy K, Tamai C, Lefebvre S, Baird G, Horvat CR, et al., Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature 1999; 379: 349–353 [PubMed]
[15].Bruno T, Iezzi S, Nicola DF, Padova DM, Desantis A, Scarsella M et al.,Che-1 activates XIAP expression in response to DNA Damage. Cell Death and Differentiation 2008[Pubmed]
[16].Huihua X, Shiying YU, Liang Z, Hua X .Changes of Survivin mRNA and Protein Expression duringPaclitaxel Treatment in Breast Cancer Cells. Journal of Huazhong University of Science and Technology 2007; 27: 65-67.
[17]Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, et al., Expression and prognostic significance of IAP family genes in human cancers and myeloid leukemias. Clinical Cancer Research. 2000; 6(5):1796-1803.
[Pubmed]
[Full text]
[18]. Schimmer AD, Dalili S. Targeting the IAP family of caspase inhibitors as an emerging therapeutic strategy. Hematology Am Soc Hematol Educ Program .2005:215-9. [PubMed]
[19]. Bilim V, Kasahara T, Hara N, Takahashi K, Tomita Y. Role of XIAP in the malignant phenotype of transitional cell cancer (TCC) and therapeutic activity of XIAP antisense oligonucleotides against multidrug-resistant TCC in vitro. Int J Cancer. 2003; 103: 29-37.
[Pubmed]
[Full text]
[20]. Alas S, Ng CP, Bonavida B. Rituximab modifies the cisplatin-mitochondrial signaling pathway, resulting in apoptosis in cisplatin-resistant non-Hodgkin's lymphoma. Clin Cancer Res. 2002; 8: 836-841.
[Pubmed]
[Full text]
[21]Evans MK, Sauer SJ, Nath S, Robinson TJ, Morse MA, Devi GR. X-linked inhibitor of apoptosis protein mediates tumor cell resistance to antibody-dependent cellular cytotoxicity. Cell Death Dis. 2016 Jan 28;7:e2073. doi: 10.1038/cddis.2015.412.
[Pubmed]
[PMC Full text]
[22]Nestal de Moraes G, Delbue D, Silva KL, Robaina MC, Khongkow P, Gomes AR, Zona S, Crocamo S, Mencalha AL, Magalhães LM, Lam EW, Maia RC. FOXM1 targets XIAP and Survivin to modulate breast cancer survival and chemoresistance. Cell Signal. 2015 Dec;27(12):2496-505. doi: 10.1016/j.cellsig.2015.09.013. Epub 2015 Sep 25 [PubMed]
[23]Ghebeh H, Al-Khaldi S, Olabi S, Al-Dhfyan A, Al-Mohanna F, Barnawi R, Tulbah A, Al-Tweigeri T, Ajarim D, Al-Alwan M. Fascin is involved in the chemotherapeutic resistance of breast cancer cells predominantly via the PI3K/Akt pathway. Br J Cancer. 2014 Oct 14;111(8):1552-61. doi: 10.1038/bjc.2014.453. Epub 2014 Aug 12.
[Pubmed]
[24]Zhang Y, Zhu J, Tang Y, Li F, Zhou H, Peng B, et al., X-linked inhibitor of apoptosis positive nuclear labeling: a new independent prognostic biomarker of breast invasive ductal carcinoma.Diagnostic Pathology. 2011; 6:49.
[Pubmed]
[PMC full text]
[25]Hofmann HS, Simm A, Hammer A, Silber RE, Bartling B. Expression of inhibitors of apoptosis (IAP) proteins in non-small cell human lung cancer. J Cancer Res Clin Oncol 2002; 128(10):554-560 [Pubmed]
[26]Hinnis AR, Luckett JC, Walker RA. Survivin is an independent predictor of short-term survival in poor prognostic breast cancer patients. Br J Cancer. 2007; 96(4):639-645. [Pubmed]
[27]Jaffer S, Orta L, Sunkara S, Sabo E, Burstein DE. Immunohistochemical detection of antiapoptotic protein X-linked inhibitor of apoptosis in mammary carcinoma. Hum Pathol. 2007; 38(6):864-870.
[Pubmed]
[28]. Xu YC, Liu Q, Dai JQ, Yin ZQ, Tang L, Ma Y, Lin XL, Wang HX. Tissue microarray analysis of X-linked inhibitor of apoptosis (XIAP) expression in breast cancer patients. Med Oncol. 2014 Mar;31(3):764. doi: 10.1007/s12032-013-0764-8. Epub 2014 Jan 21.[Pubmed]
[29]Donovan NO, Crown J, Stunell H, Hill ADK, Dermott EM, Higgins NO et al.Caspase 3 in Breast Cancer.Clinical Cancer Research 2003; 9: 738–742.[Pubmed]
[30]Vakkala M, Pa¨a¨ko¨ P, and Soini Y. Expression of caspases 3, 6, and 8 is increased in parallel with apoptosis and histological aggressiveness of the breast lesion. Br. J. Cancer 1999; 81: 592–599 [Pubmed]
[31]Zhou S, Huang Q, Zheng S, Lin K, You J, Zhang X. miR-27a regulates the sensitivity of breast cancer cells to cisplatin treatment via BAK-SMAC/DIABLO-XIAP axis. Tumour Biol. 2016 May;37(5):6837-45. doi: 10.1007/s13277-015-4500-1. Epub 2015 Dec 10.
[Pubmed]
[32]Wang C, Ju H, Shen C, Tong Z. miR-429 mediates δ-tocotrienol-induced apoptosis in triple-negative breast cancer cells by targeting XIAP. Int J Clin Exp Med. 2015 Sep 15;8(9):15648-56. eCollection 2015.[Pubmed]
[33]Ren Y, Han X, Yu K, Sun S, Zhen L, Li Z, Wang S.microRNA-200c downregulates XIAP expression to suppress proliferation and promote apoptosis of triple-negative breast cancer cells. Mol Med Rep. 2014 Jul;10(1):315-21. doi: 10.3892/mmr.2014.2222. Epub 2014 May 8.
[Pubmed]


