Review

Metabolic Syndrome (MetS) and risk of Colorectal Cancer (CRC): a Systematic
Review and Meta-analysis
1Maryam Alfa-Wali, 2Sadie Boniface, 1Anand Sharma, 1Paris Tekkis, 2Allan Hackshaw, 1Anthony Antoniou,
- 1.Imperial College London, South Kensington Campus, London SW7 2AZ; .
- 2.University College London, 90 Tottenham Court Road, London W1T 4TJ
- Submitted: Monday, May 25, 2015
- Accepted: Sunday, July 12, 2015
- Published: Sunday, July 19, 2015
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Introduction
Metabolic syndrome (MetS) is a commonly associated with cardiovascular disease and diabetes. Interest in the relationship between MetS and cancer has been evolving. The aim of this systematic review and meta-analysis is to evaluate the association between MetS and the risk of colorectal cancer (CRC).
Method
Case-control and prospective cohort studies with CRC incidence or mortality in participants with and without MetS were included in the analysis.
Results
Fifteen studies, which reported an association between MetS and CRC, were included. This comprised 12,019 cases of CRC in a total of 739,731 participants. The results showed that MetS confers a significant increase in the risk of CRC incidence (OR 1•52, 95% CI 1•33 - 1•73). When studies that did not adjust for confounders were excluded, the effect estimate was similar (OR 1.41, 95% CI 1•25 - 1•58). MetS is associated with an increased risk (51%) of CRC in both males and females.
Conclusion
It may be beneficial to identify and optimally treat MetS components as part of the screening or preventive measures for risk factor modification of CRC.
Keywords
colorectal cancer, metabolic syndrome, risk factors, meta-analysis, systematic review, colon cancer, rectal cancer
Introduction
Metabolic syndrome (MetS) is defined as a cluster of risk factors for cardiovascular disease and type II diabetes which occur together and include hyperglycaemia, hypertension, raised triglyceride levels, low high density lipoprotein (HDL) levels and central obesity
[1, 2]. An aspect of the pathophysiology of the syndrome lies with the inter-relationship between insulin and glucose resistance that is mediated though the changes in plasma concentrations of free fatty acids
[3]. It can be described as a trilogy of factors that comprise of elements such as obesity, clinical consequences such as type 2 diabetes and hypertension, as well as biochemical components in the form of lipids and glucose. The imbalance between insulin secretion and activity results in hyperinsulinaemia and hyperglycaemia, which subsequently lead to type 2 diabetes
[3].
Research into MetS was previously limited to its risk potential in medical conditions. In more recent years cancer links with MetS have been explored. Multiple studies and epidemiological data have suggested the risk of cancer in individuals with MetS
[4]. Some studies have reported an association between precursor lesions such colorectal adenomas with MetS
[5, 6]. However, the CLUE II study on the risk of colorectal adenomas with MetS did not find a statistically significant association with the individual components of MetS, with the exception of diabetes
[7]. It may be that the components of MetS become more prominent in the risk assessment of colorectal cancer (CRC) in the state of malignancy.
Specifically, obesity and MetS have been shown to be related to the development of CRC
[8, 9]. Obesity and being overweight was thought to account for over 15,000 cases of CRC in Europe in 2002
[10]. Obesity is one of the greatest public health challenges in developed countries, and as a result MetS is increasing at epidemic proportions too
[11]. The risk of gastrointestinal cancers and colorectal adenomas with excess body weight is approximately twice that of individuals with normal body weight
[12]. A previous meta-analysis shown that an increase in BMI (>27kg/m2) compared with normal BMI is associated with a relative risk (RR) of CRC of 1•19 with a 95% confidence interval (CI) of 1•11 - 1•29
[9]. MetS has been associated with an increased risk of CRC [13,
14], however the extent of this risk has varied. A systematic review 52 which combined studies of CRC and polyps or adenomas reported a pooled 34% increased risk of these outcomes among individuals with MetS. The aim of the study was to evaluate the association between MetS and CRC only, excluding precursor lesions through a systematic review and meta-analysis.
Methods
We conducted a systematic review and meta-analysis following the ‘preferred reporting items for systematic reviews and meta-analyses’ (PRISMA) guidelines
[15]. Medline, PubMed, Cochrane and Embase were searched for all published articles that mentioned MetS and CRC. Articles published between 1965 and 2015 were reviewed to identify studies that reported the association of MetS, or in the case of older studies syndromes that were indicative of the present day definition of MetS. The majority of the studies were from the last decade because MetS was only described after Reaven’s paper in 19883 and officially defined in 1998
[16]. The search terms used were ‘metabolic syndrome’, ‘insulin resistance syndrome’ or ‘syndrome X’; and ‘colorectal carcinoma/cancer/tumour/neoplasm’, ‘colon cancer/tumour/ neoplasm’ or ‘rectal cancer/tumour/ neoplasm’. The last date of the search was performed on the 15th of January 2015.
In total, 445 potentially relevant articles were identified from the literature (Figure 1). These abstracts were screened, and from these, 374 abstracts were excluded (by MA-W and AS) and the full text of 79 papers was examined. Reference lists of articles were subsequently searched manually to further identify possible relevant articles. The inclusion criteria were case-control or prospective cohort studies that reported the incidence and/or mortality of CRC in individuals with and without MetS. Articles were excluded if they were editorials or commentaries (n = 11), reviews (n = 15), adenoma studies (n = 23) or animal experiments (n = 15). Where duplicate studies were identified the most recent was selected. Fifteen studies, which reported associations between MetS and cancer in general and mentioned the associations involved with CRC, were excluded from the review.
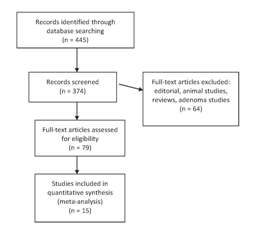
Figure1: Identification of eligible studies
In total, 15 studies were identified that matched the selection criteria for inclusion in this meta-analysis. Data was collected on the type of study, sample size, gender, definition of MetS and MetS components used. Studies were included if the incidence or mortality of CRC were associated with MetS, which reported a hazard ratio (HR), odds ratio (OR) or relative risk (RR), or if a crude OR could be calculated from data presented in the text. Where possible, we used the effect sizes adjusted for potential confounding factors and present separate analyses for these. Where studies reported on the components of MetS, the definitions based on three or more components of MetS being present were used. A number of definitions exist for MetS and were used in the various studies ( Table 1). The meta-analysis, heterogeneity tests, and assessment of publication bias were performed using the software package, Review Manager (RevMan) version 5•1
[17 18]. We did not formally assess study quality in our analyses, due to the limited number of study designs included (case-control and prospective cohort only), the general limitations of which are well-established. We identified that the key features of study quality (such as providing the definition of cases and controls, the definition of MetS, and details of adjustment for confounding factors), were present in most studies.
|
Group |
Definition criteria of Metabolic Syndrome |
|
(a) National Cholesterol Education Program / Third
Adults Treatment Panel (NCEP-ATP III).[19 20] |
The presence of 3 or more of 5
components:
·
Waist
circumference: Male ≥ 40 inches (101-102cm), female ≥ 35 inches (88cm)
·
Serum
glycerides ≥ 150mg/dl (1·7mmol/l) or taking
medication for elevated triglyceride levels
· High
density lipoprotein (HDL) < 40mg/dl (1·03mmol/l)(male), < 50mg/dl
(1·29mmol/l) (female) or taking medication for low HDL levels
· High Blood
pressure (BP) ≥ 130/85mmHg or taking medication for elevated blood pressure levels
· Fasting glucose ≥ 100-110mg (5·6mmol/l) or taking medication
for elevated blood glucose level
|
|
(b) World Health Organisation (WHO) definition of
Metabolic Syndrome factors.[16 21] |
·
Hypertension ≥ 140mmHg (systolic), ≥ 90mmHg (diastolic) or use of
anti-hypertensives 14 days prior to blood sampling
·
Impaired
fasting glucose/tolerance: fasting glucose ≥ 6·1mmol/l, post-load
glucose ≥ 8·9mmol/l
·
Raised TAGs
≥1·7mmol (150mg/dl)
And/or
·
Obesity:
Waist: hip > 0·90 (male), > 0·85 (female) and/or BMI > 30kg/m2
·
HDL: Males
<0·9mmol/l (35mg/dl), females <1.0mmol/l (39mg/dl)
·
Microalbuminuria: Urinary albumin excretion rate > 20μg/min or albumin:
creatinine ratio ≥30mg/g
|
|
(c) International Diabetes Federation (IDF).[22,23] |
·
Waist
circumference ≥ 94cm (European males), ≥ 80cm (European females)
+ any of the following:
·
Raised
triglycerides ≥ 1·7mmol/l or treatment for lipid abnormality
·
Reduced HDL
< 1·03mmol/l (males)/< 1·29mmol/l (females)
·
Raised BP:
systolic ≥ 130mmHg or diastolic ≥ 85mmHg or treatment of hypertension
·
Fasting plasma glucose ≥ 5·6mmol/l or previously diagnosed type II
diabetes
|
|
(d) Japanese Ministry of Health, Labour and
Welfare - Modified IDF.[24] |
·
Increased waist
circumference (men ≥85 cm, women ≥85cm or ≥80cm) and at least two out
of:
·
Elevated BP
(systolic BP ≥130mmHg and/or diastolic ≥85mmHg),
·
Elevated fasting
glucose level (≥110mg/dl),
·
Dyslipidaemia (HDL<40mg/dl and/or TG≥150mg/dl).
|
| (e) Diabetic Society of the Chinese Medical
Association.[25] |
The presence of ≥3
of the following:
·
Central obesity:
BMI ≥25kg/m2
·
Hypertension:
anti-hypertensive drug treatment and/or systolic BP ≥140mmHg or
diastolic BP ≥90mmHg
·
Abnormal lipids:
high TG (≥1·7mmol/l) and/or low HDL (male: <0·9mmol/l, female:
<1·0mmol/l)
·
Fasting plasma glucose: ≥6·1mmol/l or 2 hour post-prandial glucose
≥7·8mmol/l
|
| (f) Modified NCEP-ATPIII.[26] |
·
BMI ≥27kg/m2
·
Total cholesterol
≥240mg/dl or the use of lipid lowering drugs
·
BP ≥130/85mmHg or
use of anti-hypertensives
·
Diagnosis of diabetes
|
| (g) Insulin Resistance Syndrome. [27] |
Abnormal levels of serum total triglycerides, HDL
cholesterol, blood glucose and BP |
|
(h) Harmonized definition.[1]
{Joint interim statement of the IDF Task Force on Epidemiology &
Prevention; National Heart Lung & Blood Institute, American Heart
Association, World Heart Federation, International Atherosclerosis
Society and the International Association for the Study of Obesity |
Any 3 of the following:
·
Abdominal obesity: Males ≥ 94cm, females ≥ 80cm (for a
European population)
·
Elevated TAGs: ≥ 150 mg/dl (1·7mmol/l) or treatment for lipid
metabolism
·
Reduced HDL:
Males: <40mg/dl (1·03 mmol/l), female: < 50mg/dl (1·29 mmol/l) or drug
treatment
·
Fasting glucose levels ≥ 100mg/dl (≥ 5·6 mmol/l) or drug treatment
|
|
BP –
blood pressure, HDL – high-density lipoproteins, LDL – low-density
lipoproteins, TG - triglycerides |
Results
The characteristics of the 15 studies included in the meta-analysis are shown in Table 2. There were 739,731 participants in total, which included 395,867 males and 337,961 females (total does not equal the sum of men and women because some studies did not stratify by gender). There were 12,019 cases of CRC. Two studies by Colangelo [28] and Trevisan
et al., [27] analysed the effects of MetS on CRC mortality.
|
Study |
Country |
Study year(s) |
Study design |
Outcome |
Metabolic syndrome definition used |
|
Ahmed 2006 [14] |
USA |
1987-2000 |
Prospective, Population based cohort, Multicenter |
CRC incidence |
a |
|
Aleksandrova 2011 [29] |
Europe: UK, France, Denmark, Spain, Italy, Greece, Germany, the
Netherlands |
1992-2000 |
Case control (nested within European Prospective Investigation in Cancer
and Nutrition (EPIC) cohort) |
CRC incidence |
a, c, h |
|
Ashbeck 2009 [30] |
USA |
1990-2000 |
Prospective cohort (within randomized trial) |
CRC incidence |
a |
|
Bowers 2006 [31] |
Finland |
1985-2002 |
Prospective cohort (within randomized trial) |
CRC incidence |
a |
|
Chiu 2007 [14] |
Taiwan |
2004 |
Prospective |
CRC incidence |
a |
|
Colangelo 2002 [28] |
USA |
1967-1997 |
Prospective cohort |
CRC mortality |
g |
|
Kabat 2011 [32] |
USA |
1993-2010 |
Prospective, Multicenter |
CRC incidence |
a |
|
Kaneko 2010 [33] |
Japan |
2007-8 |
Prospective |
CRC incidence |
d |
|
Kontou 2012 [34] |
Greece |
2009-10 |
Case-control |
CRC incidence |
a |
|
Pelucchi 2010 [35] |
Italy & Switzerland |
1992-2001 |
Case-control |
CRC incidence |
c |
|
Shen 2009 [36] |
China |
2002-2007 |
Case-control |
CRC incidence |
e |
|
Stocks 2008 [37] |
Sweden |
1985-1996 |
Case-control (from within cohort) |
CRC incidence |
b |
|
Stocks 2011 [38] |
Europe: Norway, Sweden, Austria |
1972-2006 |
Prospective cohort |
CRC incidence |
b |
|
Sturmer 2006 [26] |
USA |
1982-2003 |
Prospective cohort (within randomized trial) |
CRC incidence |
a (and f in the absence of data needed to fulfill NCEP-ATP III
definition). |
|
Trevisan 2001 [27] |
Italy |
1978-1987 |
Prospective cohort |
CRC mortality |
g |
|
Studies used definitions of metabolic syndrome based on: a – NCEP-ATP
III, a* Modified Asian – HDL cholesterol <40mg/dl, waist circumference
>90cm in males and > 80cm in females, [39]b - WHO, c – IDF, d - Japanese
Ministry of Labour, e - Diabetic Society of the Chinese Medical
Association, f – Modified NCEP-ATP III, g - Insulin Resistance Syndrome,
h – Harmonized definition. The study by Ahmed et al[14] used the
NCEP-ATP III definition with its updated criteria of a fasting glucose
of >5.6mM in 2005 compared to ≥6.1mM in its 2001 definition.[19] |
Significant associations between MetS and CRC were observed, with an increased risk of CRC among those with MetS, although not all these individual associations were significant. Based on the 15 studies, the overall OR for CRC incidence in MetS in both genders was 1•52 (95% CI 1•33 - 1•73, p < 0•00001) (Figure 2). There was a slightly elevated risk among women with the pooled ORs for males and females being 1•35 (95% CI 1•19 - 1•53) and 1•47 (95% CI 1•17 - 1.85) respectively. Twelve studies were adjusted for confounding factors, details of which are shown in Table 3. The pooled adjusted OR for these studies alone was in line with that based on all studies (OR 1•41 (95% CI 1•25 - 1•58) (Figure 3). Again, the OR was slightly elevated in women at 1.46 (95% CI 1.15-1.84), with the OR for males at 1.34 (95% CI 1.19-1.52). The OR for CRC mortality in patients with MetS was 1•90 (95% CI 1•00 - 3•60) from the two available studies.
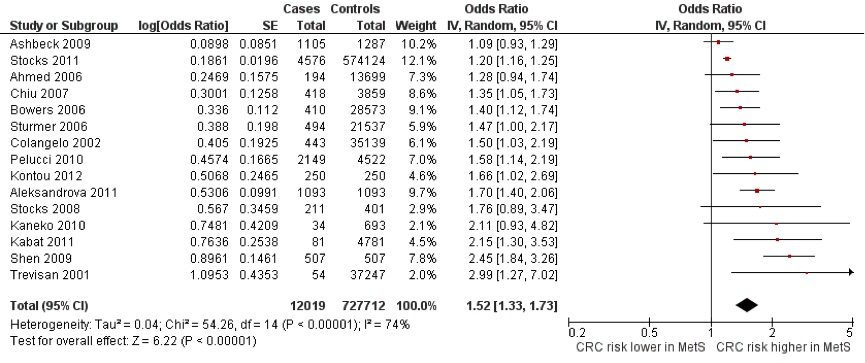
Figure 2: Forest Plot of CRC and MetS in both males and females. The OR reported for the study by Sturmer et al., [26] is based on calculated crude relative risk.
|
Study |
Adjusted OR (95% CI) of CRC in MetS |
Adjusted Variables |
|
Ahmed 2006 [14] |
1·26 (0·9-1·7) |
A, AI, AU, FH, G, NU, PA, SH |
|
Aleksandrova* 2011 [29] |
1·70 (1·40-2·06) |
AI, Ed, FI, PA, SH |
|
Ashbeck 2009 [30] |
1·09 (0·93-1·29) |
SH, AU, FH, FI, Al, PH, PA |
|
Bowers 2006 [31] |
1·40 (1·12-1·74) |
A, SH, TC |
|
Chiu 2007 [13] |
1·35 (1·05-1·73) |
A, AI, BMI, FH, G, PH, SH |
|
Colangelo 2002 [28] |
1·50 (1·03-2·19) |
A, Ed, Eth, G, H |
|
Kabat 2011 [32] |
2·15 (1·30-3·53) |
A, AI, Eth, FH, PA |
|
Kontou 2012 [34] |
1.41 (1.25-1.58) |
A, BMI, FH, G, PA, SH |
|
Pelucchi 2010 [35] |
1·58 (1·14-2·19) |
A, AI, Ed, EI, G, PA, SC, SH |
|
Stocks 2011 [38] |
1·20 (1·16-1·26) |
A, BY, SH |
|
Sturmer 2006 [26] |
1·40 (0·9-2·1) |
A, PA, SH, Al, MV, NU, FI, AH |
|
Trevisan 2001 [27] |
2·99 (1·27-7·02) |
A, AI, G, SH |
*
Pooled values
A (age), AI (alcohol intake), AU (aspirin use), BMI
(Body Mass Index), BY (birth year in cohort study), Ed (education), Eth
(ethnicity), EI (non-alcohol energy intake), FH (family history of CRC),
FI (fibre intake), G (gender), H (height), NU (NSAID use), NSAID-
non-steroidal anti-inflammatory drugs, PA (physical activity), PH
(personal history), SC (study centre in multicentre study), SH (smoking
history), TC (total cholesterol), MV (multivitamin use), AH (history of
arthritis). |
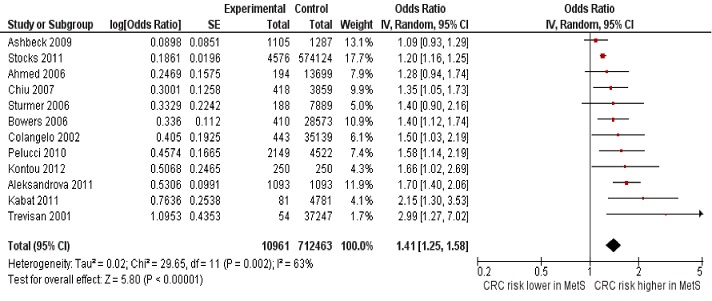
Figure 3 Forest plot of the studies that adjusted for confounding factors in both genders
Heterogeneity
The direction of the association between CRC and MetS was consistent but there was variation in the magnitude of the effect sizes. This resulted in significant heterogeneity (p < 0•00001, I2 = 74%) in the main result (Figure 2). There was more heterogeneity among the studies of females (p = 0•002, I2 = 66%) than the male studies (p = 0•05, I2 = 46%). Similar values for heterogeneity were found when this was restricted to studies that reported adjusted ORs: male and female studies (p = 0•002; I2 = 65%), male only studies (p = 0.06, I2 = 45%), and female studies (p = 0•002, I2 = 70%). This heterogeneity can be attributed to the different study designs, MetS definitions and CRC definitions included in the meta-analysis. However, due to the small number of studies, subgroup analysis by these particular features would not be reliable and was therefore not conducted.
Sensitivity Analysis
There were no obvious outliers identified in terms of effect sizes. The funnel plot (Figure 4) is not symmetrical suggesting there may be some publication bias in studies of MetS and CRC, with smaller studies reporting only a small or no association less likely to be published. Smaller studies with the largest standard errors (Stocks 2008
[37], Kaneko 201033 and Trevisan 2001[27] had ORs in line with other larger studies. When these studies are excluded from the analysis, the OR for CRC in both genders was similar at 1•44 (95% CI 1•25 - 1•65).
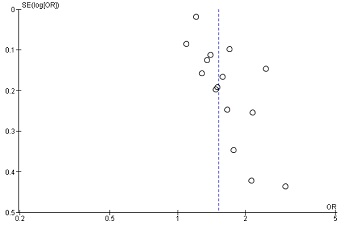
Figure 4 Funnel plot of the studies. The lack of symmetry among the smaller studies with larger SEs indicates publication bias. SE – standard error, OR – odds ratio.
Discussion
This systematic review and meta-analysis focuses on confirmed cases of CRC and the significant with increased risk of CRC (OR 1•51, 95% CI 1•32 - 1•73) with MetS. There was a suggestion that the CRC risk is greater among females with MetS (OR 1•47, 95% CI 1•17 - 1•85) than males (OR 1.35, 95% CI 1•19 - 1•53); but this difference is not great and the confidence intervals overlap. Some of the individual studies however have indicated that the association between MetS and CRC risk is greater in males
[14, 27, 40, 35, 41, 42]. Only the study by Kabat
et al., [32] in this meta-analysis concentrated on post-menopausal women, as other studies reported on either mixed genders or predominantly a male population.
The Metabolic syndrome and Cancer project (Me-Can) [43] was set up over a number of European countries to investigate the relationship between MetS and cancer risk. This study involved over 4000 participants, and found a smaller RR of 1•25 (95% CI 1•18 - 1•32) in males and 1•14 (95% CI 1•06 - 1•22) in females for CRC
[38]. Different reasons have been reported for the discrepancy in incidence between males and females with CRC and MetS. The Me-Can study revealed significant associations for high BMI, blood pressure and triglycerides for males. However for females BMI was observed as a significant factor
[38]. The Kabat and co-workers [32] women’s study on the other hand showed that the associations between CRC and MetS were mostly due to hyperglycaemia and hypertension. Recently, associations of MetS with aggressive CRC phenotypes in males has been observed by Healy and colleagues
[44]. Hypertension and high cholesterol have been observed to be more associated with male colon cancer than rectal cancer
[35].
Tal and colleagues reported MetS being a risk factor for advanced polyps, which they defined as a villous ≥ 1cm or with high-grade dysplasia[45]. Apart from MetS, multiple metabolic pathways may be involved in the pathogenesis of CRC. Insulin resistance in particular has been thought to be one of the main drivers of cancer risk in MetS, as well as the main underlying cause of MetS
[46]. This is due to the increase in insulin secretion and the subsequent hyperinsulinaemia activating the insulin receptor, insulin-like growth factor-1 (IGF-1)
[13]. Some studies have shown that being overweight with diabetes to be associated with increased risk of CRC
[26, 47]. Aleksandrova et al., [29] and Stocks
et al., [37] found that hyperglycaemia was associated with an increase in CRC and accounted for the association between CRC and MetS. The rationale for studying diabetes as a risk factor for CRC has been following in vitro studies that insulin can stimulate tumour growth.[48] A meta-analysis of diabetes CRC found a RR of 1•30 (95% CI 1•20 - 1•40) for CRC incidence and a RR of 1•26 (95% CI 1•05 - 1•50) for CRC mortality
[49].
The mechanism by which MetS increases the risk of CRC as well as other cancers is yet to be elucidated, as it is a multi-faceted disorder. The gut microflora which has been referred to as a “microbial organ” by Cani
et al., [50] may also play a role in the pathophysiology of MetS and CRC. The changes in gut microbes have been associated with obesity in animal models
[51]. Decreases in Bacteroides species have been observed in obese individuals compared with individuals with normal BMI
[52]. Furthermore, the inter-relationship between the rise in free fatty acids and insulin resistance through the activity of adipokines such as interleulin-6 (IL-6) and tumour necrosis factor- alpha (TNF-α) are thought to be mediators in cancer biology. Hence, the hyperinsulinaemic state leads to increases in IGF-1 which subsequently lead to the pro-tumorigenic state
[2, 53, 54, 55, 56] The extent of the role of obesity in MetS and CRC is still controversial. Obesity in the forms of a high BMI or large waist circumference seem to be reported as major contributors to CRC as reported by a number of studies [9]. Stürmer and co-workers [56] reported that a BMI of ≥ 27 kg/m2 and diabetes were independently associated with an increased risk of CRC (HR 1•4 (95% CI 1•1 - 1•7) and 1•5 (95% CI 1•1 - 2•0) respectively). Kaneko
et al., [33] and Healy et al., [44] also reported that a larger waist circumference of >80cm in females increased the OR for both colonic adenomas and carcinoma. Other authors have found no association between BMI and CRC risk [47, 57]. Interestingly, the Mediterranean diet has been shown to have a protective effect against high-risk CRC patients with MetS
[34].
Chiu et al., [13] observed a stronger effect of MetS on proximal rather than distal tumours. This may have implications in future screening programmes, which may need to incorporate MetS screening in the investigations for microcytic anaemia thought to be due to colonic pathology. A recent clinical study reported having MetS to have a higher rate of post-operative complications after CRC surgery
[58]. Additionally, the rate of liver metastasis and recurrence of CRC in patients that have MetS was thought to be higher (HR of 4•77 (95% CI 1•58 - 14•35))
[36]. Nevertheless, Yang et al., [59] concluded from a retrospective patient cohort that MetS has no effect on colon cancer recurrence or survival.
Historically, the main reason for identifying MetS was to identify individuals at risk of developing cardiovascular disease or diabetes. This review shows detecting patients with MetS could also identify those at risk of CRC. Early diagnosis of CRC is fundamental to long term survival; therefore the identification of MetS could potentially reduce morbidity and mortality. In particular, managing the components of MetS optimally could aid in the decreasing the metabolic effects associated with cancer. Chemo-preventive approaches using NSAIDs in CRC may be related to the association of CRC and MetS as conditions such as hypertension and the use of aspirin as a prophylactic measure for cardiovascular events. Studies have also reported the benefit of aspirin in CRC patients [
60]. As obesity is increasingly becoming a global epidemic, weight loss programmes should also become part of the cancer prevention strategy as a component of MetS management. Since, weight loss has been associated with a decrease in incidence of CRC in large cohort studies
[
61,
62].
One limitation of our meta-analysis is the multiple definitions of MetS used in various studies. This makes comparisons of study populations challenging, as different definitions place an importance on certain components of MetS aside from variations in cut-offs for parameters used. A universal definition of MetS that can be used by all clinicians in practice is still in progress. Nonetheless, attempts have been made to unify the multiple definitions of MetS with a Harmonized definition [1] involving multiple learned societies, although this definition is yet to be fully embraced.
Other confounding factors are the different study populations, which may have an influence on biology as well as unmeasured environmental factors even though their outcomes may be the same for CRC. The biochemical measurements may not be necessarily comparable, as different methods may have been used in different countries. There are also random measurement errors from the different studies when taking measurements of blood pressure and waist circumference. However, three studies by Kabat, [32] Stocks [38] and Strumer
et al., [26] used repeated measurements in order to minimise errors. There are also presently no universally acceptable cut offs for waist-hip ratio measurements and this may be difficult to develop on a universal global level due to different levels at which different populations experience adverse effects. Only two studies reported on CRC mortality so this effect estimate needs to be treated with caution. Additionally, the CRC stage was not taken into consideration for the analysis, as most studies did not report on this in relation to MetS. What lies beyond this meta-analysis is the aetiology of MetS in the first place before it begins to place a role in the manifestation of other diseases such as cancer.
Conclusions
This systematic review and meta-analysis suggests shows that there is a significant association between having MetS and the risk of CRC. Therefore, preventing and controlling the components of MetS could be important, changes in lifestyle and dietary habits. The exact mechanism how MetS relates to CRC pathophysiology is yet to be clarified and we suspect that MetS is more likely to precede the diagnosis of CRC, rather than the malignancy itself having a direct effect on lipid levels. Understanding the pathophysiology of MetS would enable the development of therapeutic and preventative strategies in relation to the CRC, which could be made at a personalised level by identifying individuals at greatest risk.
Funding
None
Conflict of Interest
We have not received financial support or have conflicts of interests to declare
Author contributions
Concept of study: M-AW, AA, PT; Data collection: M A-W, SB, AS Data analysis: SB, M A-W, AH Writing of manuscript: M A-W, AS, SB; Review of manuscript: All authors
References
[1]. Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the Metabolic Syndrome. Circulation 2009;120(16):1640-45.[Pubmed]
[2]. Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocrine reviews 2008;29(7):777-822.[Pubmed]
[3]. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37(12):1595-607.[Pubmed]
[4]. Russo A, Autelitano M, Bisanti L. Metabolic syndrome and cancer risk. European Journal of Cancer 2008;44(2):293-7. [Pubmed].
[5]. Kim JH, Lim YJ, Kim YH, et al. Is metabolic syndrome a risk factor for colorectal adenoma? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2007;16(8):1543-6.
[6]. Wang YY, Lin SY, Lai WA, et al. Association between adenomas of rectosigmoid colon and metabolic syndrome features in a Chinese population. Journal of gastroenterology and hepatology 2005;20(9):1410-5. [Pubmed].
[7]. Tsilidis KK, Brancati FL, Pollak MN, et al. Metabolic syndrome components and colorectal adenoma in the CLUE II cohort. Cancer causes & control : CCC 2010;21(1):1-10. [Pubmed].
[8]. Pais R, Silaghi H, Silaghi AC, et al. Metabolic syndrome and risk of subsequent colorectal cancer. World journal of gastroenterology : WJG 2009;15(41):5141-8. [Pubmed].
[9]. Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2007;16(12):2533-47
[10]. Renehan AG, Soerjomataram I, Tyson M, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. International journal of cancer Journal international du cancer 2010;126(3):692-702.[Pubmed].
[11]. Grundy SM. Metabolic syndrome pandemic. Arteriosclerosis, thrombosis, and vascular biology 2008;28(4):629-36.[ [Pubmed].
[12]. Kant P, Hull MA. Excess body weight and obesity--the link with gastrointestinal and hepatobiliary cancer. Nature reviews Gastroenterology & hepatology 2011;8(4):224-38. [Pubmed].
[13]. Chiu HM, Lin JT, Shun CT, et al. Association of metabolic syndrome with proximal and synchronous colorectal neoplasm. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2007;5(2):221-9; quiz 141.
[14]. Ahmed RL, Schmitz KH, Anderson KE, et al. The metabolic syndrome and risk of incident colorectal cancer. Cancer 2006;107(1):28-36.[Pubmed]
[15]. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology 2009;62(10):1006-12.
[16]. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic medicine : a journal of the British Diabetic Association 1998;15(7):539-53.
[17]. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine 2002;21(11):1539-58.[Pubmed]
[18]. Review Manager (RevMan) [program]. Copenhagen: The Nordic Cochrane, 2011.
[19]. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112(17):2735-52. [Pubmed].
[20]. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA : the journal of the American Medical Association 2001;285(19):2486-97.
[21]. Organisation WH. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Part 1: Diagnosis and classificationof diabetes mellitus. Geneva, 1999.
[22]. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic medicine : a journal of the British Diabetic Association 2006;23(5):469-80.[Pubmed].
[23]. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet 2005;366(9491):1059-62.[Pubmed].
[24]. Kaneko R SY, An Y, Nakagawa M, Kusayanagi S, Kamisago S, Umeda T, Ogawa M, Munakata K, Mizuno K. Clinico-epidemiologic study of the metabolic syndrome and lifestyle factors associated with the risk of colon adenoma and adenocarcinoma. Asian Pac J Cancer Prev 2010;11(4):975-83. [Pubmed].
[25]. Shen Z, Wang S, Ye Y, et al. Clinical study on the correlation between metabolic syndrome and colorectal carcinoma. ANZ journal of surgery 2010;80(5):331-6.[Pubmed].
[26].Sturmer T, Buring JE, Lee IM, et al. Metabolic abnormalities and risk for colorectal cancer in the physicians' health study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2006;15(12):2391-7.
[27].Trevisan M, Liu J, Muti P, et al. Markers of insulin resistance and colorectal cancer mortality. Cancer epidemiology, biomarkers& prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2001;10(9):937-41.
[28].Colangelo LA, Gapstur SM, Gann PH, et al. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2002;11(4):385-91.
[29].Aleksandrova K, Boeing H, Jenab M, et al. Metabolic syndrome and risks of colon and rectal cancer: the European prospective investigation into cancer and nutrition study. Cancer prevention research 2011;4(11):1873-83 [Pubmed]
[30].Ashbeck EL, Jacobs ET, Martinez ME, et al. Components of metabolic syndrome and metachronous colorectal neoplasia. Cancer epidemiology, biomarkers &prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2009;18(4):1134-43.
[31].Bowers K, Albanes D, Limburg P, et al. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. American journal of epidemiology 2006;164(7):652-64.
[32].Kabat GC, Kim MY, Peters U, et al. A longitudinal study of the metabolic syndrome and risk of colorectal cancer in postmenopausal women. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation 2012;21(4):326-32.
[33].Kaneko R, Sato Y, An Y, et al. Clinico-epidemiologic study of the metabolic syndrome and lifestyle factors associated with the risk of colon adenoma and adenocarcinoma. Asian Pacific journal of cancer prevention : APJCP 2010;11(4):975-83.
[34].Kontou N, Psaltopoulou T, Soupos N, et al. Metabolic syndrome and colorectal cancer: the protective role of Mediterranean diet--a case-control study. Angiology 2012;63(5):390-6 [Pubmed]
[35].Pelucchi C, Negri E, Talamini R, et al. Metabolic syndrome is associated with colorectal cancer in men. European journal of cancer 2010;46(10):1866-72 [Pubmed]
[36]Shen Z, Ye Y, Bin L, et al. Metabolic syndrome is an important factor for the evolution of prognosis of colorectal cancer: survival, recurrence, and liver metastasis. American journal of surgery 2010;200(1):59-63 [Pubmed]
[37].Stocks T, Lukanova A, Johansson M, et al. Components of the metabolic syndrome and colorectal cancer risk; a prospective study. International journal of obesity 2008;32(2):304-14. [Pubme]
[38].Stocks T, Lukanova A, Bjørge T, et al. Metabolic factors and the risk of colorectal cancer in 580,000 men and women in the metabolic syndrome and cancer project (Me-Can). Cancer 2011;117(11):2398-407 [Pubmed]
[39].Tan CE. Can We Apply the National Cholesterol Education Program Adult Treatment Panel Definition of the Metabolic Syndrome to Asians? Diabetes Care 2004;27(5):1182-86 [Pubmed]
[40]Bowers K, Albanes D, Limburg P, et al. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am J Epidemiol 2006;164(7):652-64 [Pubmed]
[41].Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. The American journal of clinical nutrition 2007;86(3):s836-42 [Pubmed]
[42].Ashbeck EL, Jacobs ET, Martinez ME, et al. Components of metabolic syndrome and metachronous colorectal neoplasia. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2009;18(4):1134-43.
[43].Stocks T, Borena W, Strohmaier S, et al. Cohort Profile: The Metabolic syndrome and Cancer project (Me-Can). International journal of epidemiology 2010;39(3):660-7 [pubmed]
[44].Healy L, Howard J, Ryan A, et al. Metabolic syndrome and Leptin are Associated with Adverse Pathological Features in Male Colorectal Cancer Patients. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland 2011.
[45].al S, Melzer E, Chsherbakov T, et al. Metabolic syndrome is associated with increased prevalence of advanced colorectal polyps. The journal of nutrition, health & aging 2014;18(1):22-5 [pubmed]
[46].Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. The American journal of clinical nutrition 2007;86(3):s836-42. [Pubmed]
[47].Schoen RE, Tangen CM, Kuller LH, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. Journal of the National Cancer Institute 1999;91(13):1147-54 [Pubmed]
[48].Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? Journal of cell science 2001;114(Pt 16):2903-10 [Pubmed]
[49].Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. Journal of the National Cancer Institute 2005;97(22):1679-87.[Pubmed]
[50].Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des 2009;15(13):1546-58 [Pubmed]
[51].Ley RE, Backhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005;102(31):11070-5.[Pubmed]
[52].Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature 2006;444(7122):1022-3.[Pubmed]
[53].Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocrine reviews 2005;26(3):439-51.[Pubmed]
[54].Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. The Proceedings of the Nutrition Society 2001;60(1):91-106 [Pubmed]
[55].Giovannucci E. Insulin and colon cancer. Cancer causes & control : CCC 1995;6(2):164-79.[Pubmed]
[56].Bjorntorp P. "Portal" adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 1990;10(4):493-6.[Pubmed]
[57].Nilsen TI, Vatten LJ. Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose and BMI: exploring the hyperinsulinaemia hypothesis. British Journal of Cancer 2001;84(3):417-22.
[58].Lohsiriwat V, Pongsanguansuk W, Lertakyamanee N, et al. Impact of metabolic syndrome on the short-term outcomes of colorectal cancer surgery. Dis Colon Rectum 2010;53(2):186-91 [Pubmed]
[59].Yang Y, Mauldin PD, Ebeling M, et al. Effect of metabolic syndrome and its components on recurrence and survival in colon cancer patients. Cancer 2013;119(8):1512-20 [Pubmed]
[60].Bosetti C, Rosato V, Gallus S, et al. Aspirin and cancer risk: a quantitative review to 2011. Annals of Oncology 2012;23(6):1403-15[pubmed]
[61].Parker ED, Folsom AR. Intentional weight loss and incidence of obesity-related cancers: the Iowa Women's Health Study. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 2003;27(12):1447-52.
[62].Rapp K, Klenk J, Ulmer H, et al. Weight change and cancer risk in a cohort of more than 65,000 adults in Austria. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2008;19(4):641-8.[Pubmed]


