Case Report

Glioblastoma Multiforme (GBM) with Cervical Lymph Node and Skeletal Metastases: A Case Report and Review of Literature
1 Azhar Rashid, 1 Muhammad Adeel Ilyas, 1 Khalique ur Rehman, 2Mudassar Hussain, 1 Irfan Haider,1 Tahir Mehmood, 1 Arif Jamshed, 1Shahid Hameed
- 1 Radiation Oncology Department, ShaukatKhanum Memorial Cancer Hospital &Research Center Lahore Pakistan.
- 2Pathology Department, ShaukatKhanum Memorial Cancer Hospital &Research CenterLahore Pakistan.
- Submitted: October 31, 2014
- Accepted: March 1, 2015
- Published: March 7, 2015
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Glioblastoma Multiforme (GBM) is the most aggressive intracranial tumor and diffusely infiltrates the surrounding brain tissue. Despite their malignant nature, they do not typically invade blood vessels and rarely spreads outside the central nervous system (CNS). Median survival of GBM patients after completing standard treatments is about 14 months and few have long term survival. Extra neural metastases usually occur after surgery in which the tumor cells may find an access to extra-cranial vessels, the most common sites being pleura and the lungs. Although the exact mechanism of extra-neural metastasis has been poorly understood, the lymphatic drainage, the venous system and the adjacent dura and bone invasion have been suggested as the three possible routes of extra-neural spread. Interestingly, we treated a rare case of extra-neural metastases of the GBM having left neck nodal mass and osseous metastases first time at our center. She was 21 year old female who had
biopsy proven cervical lymph node metastases and radiologic evidence of skeletal metastases from Glioblastoma Multiforme and reviewed the literature.
Key Words
GBM, Metastases, Skeletal metastases
Introduction
Glioblastoma Multiforme (GBM) is the most aggressive primary
brain tumor and it commonly spreads by direct extension and infiltration into
the adjacent brain tissue and along the white matter tract rather than by
metastases. Intracranial tumors rarely give rise to extra-neural metastases [1].
First report was published in 1928 about extra-neural metastases from GBM and
other gliomas[2]. Glioblastoma Multiforme (GBM) is the most
common extra-cranially metastasizing tumor in adults and medulloblastoma the
most common in children. GBM is associated with a strong tendency for local
invasiveness, the metastatic spread of the GBM outside of the CNS is extremely
rare, occurring in 0.2- 2% of all GBMcases[3 4 5]
and accounting for about two thirds of the neuroepithelial tumors that
metastasize extra-neurally. Cervio et al., [6]
described the most frequent extra-cranial sites of metastases distribution was
60% in lungs and pleura, 51% lymph nodes, 31% bones, and 22% in liver. Among the
lymph node metastases, 62% were situated in the cervical areas, often
ipsilateral to the site of craniotomy but sometimes bilateral. In skeletal
metasteses most frequent site of involvement was vertebral spine (73%), followed
by the ribs, sternum, skull and acetabulum (23%, 18%,14% and 9% respectively) [7].
Case Report
A 21 year old female had one month history of headache and vomiting. MRI Brain showed space occupying lesion in the left temporo-parietal region which on biopsy turned out to be
astrocytoma grade III. Craniotomy was performed and a postoperative MRI scan showed residual disease for which patient had gamma knife radiosurgery for the remnant mass, with a marginal dose of 12 Gy to the 50% isodose line. She remained well for about 1 year, and then
was found to have multifocal recurrence on follow-up MRI Brain (Figure 1).
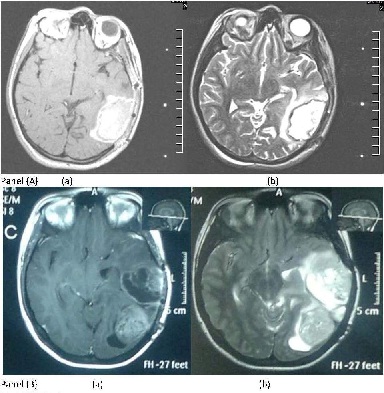
Figure 1: MRI Brain: Panel A (a) Axial T1 with contrast and (b) Axial T2 at initial diagnosis; Panel B (a) Axial T1 with contrast (b)axial T2 images at the time of recurrence.
A repeat craniotomy revealed Glioblastoma Multiforme Grade IV. After the operation, the patient made good recovery having ECOG performance status 0. At this stage patient presented to our hospital for further management. External beam radiation therapy (6000 cGy/30 Fr at 200cGy/day) alongwith concurrent temozolamide75mg/m2 daily was offered and found to have excellent response to the therapy both clinically and radiologically (Figure 2) .
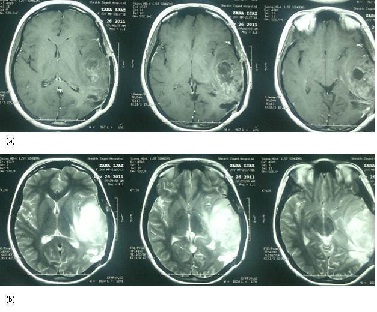
Figure 2: Post Radical Chemo-radiation therapy (a) T1 weighted images with contrast (b) T2 weighted images showing improvement.
Four weeks after concurrent chemoXRT she was started on adjuvant temozolamide 150-200 mg/m2 day 1 to day 5 every 28 days to complete 6 cycles. Soon after finishing her first cycle she developed palpable left sided level II cervical lymphadenopathy which didn’t respond to antibiotics. Mass was biopsied and the histopathology indicated a metastatic deposit of the GBM confirmed with immunohistochemistry (GFAP positive, Ki67 >30%, Cytokeratin, Desmin and LCA negative) (Figure 3, 4).
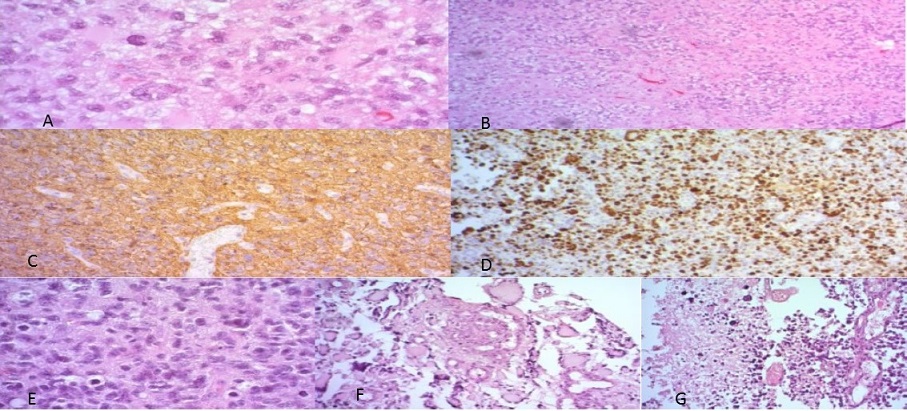
Figure 3: Photomicrographs showing A) and B) Presence of pleomorphism, high cellularity and absence of microvascular proliferation and necrosis, at initial presentation of Anaplastic Astrocytoma; C) showing GFAP positivity D) KI 67 positivity of 40% E) Cellular atypia and pleomorphism F) Microvascular proliferation and G) Necrosis
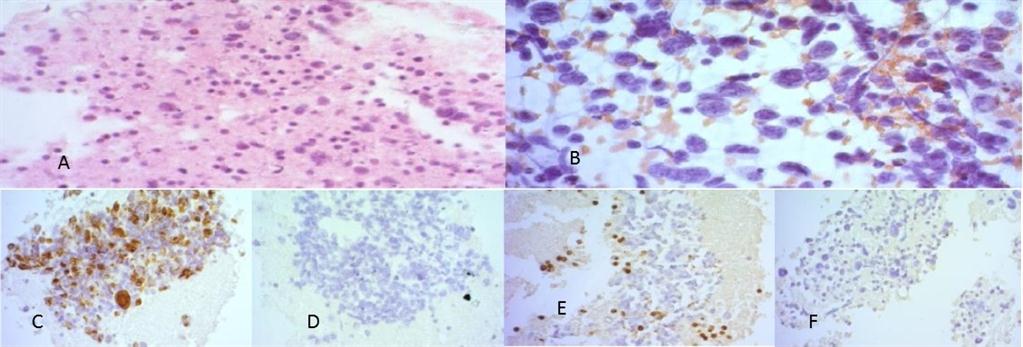
Figure 4: Photomicrograph from neck showing A) cell block preparation (H & E); B) Papanicolau staining of the smear C) GFAP positivity in cells D) Desmin negative E) LCA negative F) CK negative smears.
CT Neck & Chest revealed multiple skeletal metastases, multilevel lytic vertebral lesions with spinal cord compression at D10/D11 (Figure 5) along with left neck lymphadenopathy. Her bone scan was performed and showed irregular uptake at D10/11 and various other levels corresponding with lytic lesions in CT, which was labeled as osseous metastases. MRI thoracic spineconfirmed cord compression at D10-D11 (Figure 6) while MRI brain showed stable disease.
The case was discussed in departmental meeting and palliative external beam radiation therapy to left sided neck and to the involved thoracic spine followed by PCV (i.e. procarbazine, lomustine, and vincristine) palliative chemotherapy was planned. She received 3000 cGy in 10 fractions to each site. MRI cervico-thoracic spine showed reduction in left neck lymphadenopathy and MRI brain again showed stable disease. Later she was started on first cycle of PCV chemotherapy but she developed multilevel cord compression andacute pancreatitis. Her performance status was going down because of parapareses secondary to cord compression, immobility and new onset of acute pancreatitis. CT brain showed recurrent progressive contrast enhanced nodularity in the brain. So
chemotherapy was stopped at this stage and she was kept on best supportive care. Despite the aggressive multimodality treatment for the GBM, the patient died at 30 months after first surgery.
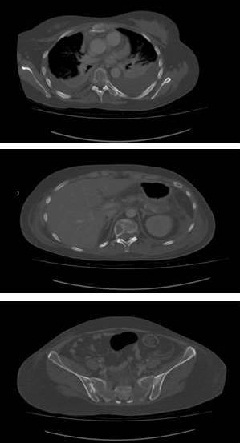
Figure 5: CT Scan showing multiple Lytic lesions at various levels of vertebral column and other bones.
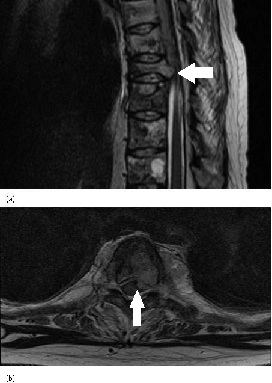
Figure 6: MRI Thoracic Spine (a) T2 weighted sagittal view confirming cord compression at D10-D11(b) T2 weighted axial view- also showing vertebral disease with cord compression.
Discussion
It is important to note that extra-neural metastases from GBMs are so rare despite the highly locally invasive nature
[8]. A variety of hypotheses have been suggested to explain the rarity of extraneural spread of glioblastomas. The first hypothesis was suggested on the basis of poor survivals of patients. The highly aggressive nature of GBM results in a very short survival period of patients and eliminates the chances of detection of metastasis from GBM [9].
Currently, new therapeutic approaches have prolonged the overall survival to
some extent, which has increased the risk of extraneural dissemination of CNS
tumors [7 10]. The second suggestion
based on the concept of capillary basement membrane barrier effect which plays
an important role as a physical barrier against migration of the glioma cells
into the blood stream [11]. Other suggestions include,
relatively no trafficking through the dura by the extracellular matrix and by
the lack of true lymphatics in the brain [1].
Although the exact mechanism of extra-neural metastases has
been poorly understood, Cervio et al., [6] suggested three possible mechanisms of extra-neural spread of GBM, as follows: 1) Lymphatic invasion: Cerebrospinal fluid drainage into the extraneural tissue (despite absence of true lymphatic system in the CNS); 2) Venous invasion: either via the leptomeningeal sinuses or via the intracerebral vein; 3) Direct invasion through the dura and bone or through tumor cell migration along the ventriculoperitoneal shunts. Romero-Rojas EA
et al., [12] described direct invasion like VP shunting, biopsy procedures, and previous surgical intervention to treat the primary tumor causes defect in meningeal and parenchymal blood vessels are created has been postulated to be a major mechanism to facilitate the extra-cranial dissemination of GBM.
In our case, the patient underwent multiple craniotomies and
radiation therapy so any above explanation can be considered as the cause of
distant metastases. However, these factors do not explain the occurrence of
extraneural metastases in all patients, and there are reports of lymph node or
distant metastases before surgery [7 13].
Following are the simple points to highlight the presence of
extra-neural metastases: 1) the clinical history must be indicative of CNS tumor
as the initial neoplasm; 2) primary CNS tumor and metastatic lesion must be
histologically identified as CNS tumor; 3) the morphologic features of the
primary lesion and of the metastatic lesion must be identical. Fine needle
aspiration cytology (FNAC) is a simple and reliable diagnostic method if such
lesions are suspected from the history [14 15]. Routine cytological examination or routine light microscopy of a biopsy specimen can give us the information on metastases from glial tumor. Ates at al. [16]
showed immunohistochemistry pattern of glioblastoma multiform at nodal site as
follows; glial fibrillary acidic protein (GFAP), S-100 protein andvimentin
positive, while tumor cells were negative for desmin, neurofilament,
synaptophysin, p53, endothelial growth factor receptor and smooth muscle actin(
leukocyte common antigen (LCA). Mujtaba et al., [17] also revealed that metastatic node was positive in GFAP and vimentin antibody, and was negative for CKAE1/AE3, CK-7, CK-20, CK-5/6 and HMB-45. While in our patient neck nodal tumor mass had GFAP positive, Ki67 was >30% while desmin, LCA and CKwere negative.
At presentation with extraneural metastasis, this patient developed enlargement of the cervical lymph node. Since the patient showed the lymphatic involvement of the GBM as extraneural metastasis, we considered that the main pathway of extraneural metastasis might be the lymphatic spread. However, we could not exclude the hematogenous spread because the patient had undergone multiple craniotomies and that may be the cause of skeletal metastases. Skeletal metastases were lytic in nature as per CT findings that correlates with Haddon M et al., [18]description of osseous metastases from GBM on plain radiographs as osteolytic and expansile lesions.
The presence of extraneural metastases does not significantly
affect the already dismal prognosis of recurrent GBM, but appropriate therapy
may increase the quality of life. Detection of extra-neural metastases is
important by itself for prediction of short survival; only palliative therapy is
available when they cause symptoms. In routine follow-up imaging of brain,
neuroradiologist should not omit a careful assessment of the extra-cranial
structures visible on follow-up brain imaging [19]. An
assessment of more discrete areas of tumor in extraneural sites such as bone can
be treated with focal radiation. We offered palliative irradiation to neck and
spine, she got good regression of nodal mass and pain relief. Systemic
chemotherapy has shown responses in extra-cranial metastasis in nodal disease
and other soft tissues [15 20 21]. Only one course of PCV chemotherapy was given in this case, so
we couldn’t assess the effect of chemotherapy on metastases adequately.
Our patient was young and has secondary GBM (GBM with
histopathalogic evidence of a precursor low grade or anaplastic astrocytoma [22 23).
P53 mutation with LOH on chromosome17p in this patient is the likely
possibility, as p53 mutation has been reported in more than 65% of secondary GBM
[24] and is associated with younger age group and better survivals than primary GBM patients. Unfortunately, we
did not have the facility to get p53 mutation testing. Moreover secondary GBM patients have better survivals than the primary GBM patients.
Conclusions
Although the exact mechanism of the extra-neural metastases is not well understood, the GBM can metastasize to extraneural organs. Longer survival period, damage to the cerebral vessels and blood brain barrier by repeated surgical manipulations could be considered as risk factors of the extra neural metastases. P53 mutation should be carried out in younger patients with diagnosis of secondary GBM to predict the survivals.
Authors’ Contributions
AR: Conceived the study, design it and wrote the manuscript.
MAL: Editing of introduction part and figures preparation.
KR: Editing and review of discussion part.
MH: Pathologist findings, figures and manuscript writing of pathology part.
IH: Editing and review of case report part
TM: Literature search and writing of introduction part
AJ: Literature review and discussion part editing.
SHEditing and review of final manuscript.
All authors read and approved the final manuscript for publication.
Conflict of Interest
The authors declare that there are no conflict of interest
Ethical Considerations
Written informed consent was obtained from the patient for publication of this case report.
Funding
None declared
References
[1].Young JS Won CH, Dong KW, Seung CH: Extraneural metastasis of glioblastoma multiforme presenting as an unusual neck mass. J Korean NeurosurgSoc 51:147-150, 2012 [Pubmed]
[2].Davis L: Spongioblastomamultiforme of the brain. Ann Surg 87:8-14, 1928 [Pubmed]
[3].Hsu E, Keene D, Ventureyra E, Matzinger AM, Jimenez C, Wang HS, et al: Bone marrow metastasis in astrocytic gliomata. J Neurooncol 37:285-293, 1998 [Pubmed]
[4].Mujic A, Hunn A, Taylor AB, Lowenthal MR. Extracranial metastases of a glioblastoma multiforme to the pleura, small bowel and pancreas. J ClinNeurosci 13:677–681, 2006 [Pubmed]
[5].Mondin V, Ferlito A, Devaney KO, Woolgar AJ, Rinaldo A: A survey of metastatic central nervous system tumors to cervical lymph nodes. Arch Otorhinolaryngol 267:1657–1666, 2010 [Pubmed]
[6].Cervio A, Piedimonte F, Salaberry J, Alcorta CS, Salvat J, Diez B, et al: Bone metastases from secondary glioblastoma multiforme: a case report. J Neurooncol 52:141–148, 2001 [Pubmed]
[7].Pasquier B, Pasquier D, N’golet A, Panh HM, Couderc P: Extraneural metastases of astrocytomas and glioblastomas: clinicopathological study of two cases and review of literature. Cancer 45:112-125, 1980
[8].Piccirilli M, Brunetto GM, Rocchi G, Giangaspero F, Salvati M: Extra central nervous system metastases from cerebral glioblastoma multiforme in elderly patients: clinico-pathological remarks on our series of seven cases and critical review of the literature. Tumori 94:40-45, 2008
[9].Mentrikoski M, Johnson DM, Korones DN, Scott AG: Glioblastoma multiforme in skin: a report of 2 cases and review of the literature. Am J Dermatopathol 30:381-384, 2008 [Pubmed]
[10].Fabi A, Vidiri A, Carapella C, Pace A, Occhipinti E, Caroli F, et al: Bone metastasis from glioblastoma multiforme without central nervous system relapse: a case report. Anticancer Res 24:2563-2566, 2004 [Pubmed]
[11].Bernstein JJ, Woodard AC: Glioblastoma cells do not intravasate into blood vessels. Neurosurgery 36:124-132, 1995 [Pubmed]
[12].Romero-Rojas AE, Diaz-Perez AJ, Amaro D, Lozano-Castillo A, Chinchilla-Olaya IS: Glioblastoma metastasis to parotid gland and neck lymph nodes: fine-needle aspiration cytology with histopathologic correlation. Head Neck Pathol 7:409–415, 2013
[13].Hoffman HJ, Duffner KP: Extraneural metastases of central nervous system tumors. Cancer 56:1778–1782, 1985 [Pubmed]
[14].Cartwright DM, Miller RT, Nasr AJ: Fine-needle aspiration biopsy of pituitary carcinoma with cervical lymph node metastases: a report of two cases and review of the literature. DiagnCytopathol 11:68–73, 1994
[15].Moon KS, Jung S, Lee CM, Kim IY, Kim HW, Lee JK, et al: Metastatic glioblastoma in cervical lymph node after repeated craniotomies: report of a case with diagnosis by fine needle aspiration. J Korean Med Sci 19:911–914, 2004 [Pubmed]
[16].Ates EL, Bayindir C, Bilgic B, Karasu A: Glioblastoma with lymph node metastases. Neuropathology 23:146–149, 2003 [Pubmed]
[17].Mujtaba SS, Haroon S, Faridi N. Cervical metastatic glioblastoma multiforme. J Coll Physicians Surg Pak 23:160-161, 2013 [Pubmed]
[18].Haddon M, Slavin DJ, Spencer PR. Multiple bone metastases in a patient with glioblastoma multiforme. ClinNucl Med 14:13-14, 1989 [Pubmed]
[19].Wallace CJ, Forsyth AP, Edwards DR: Lymph node metastases from glioblastoma multiforme. AJNR Am J Neuroradiol 17:1929–1931, 1996 [Pubmed]
[20]Salcman M: Glioblastoma multiforme and anaplastic astrocytoma, in Kaye AH, Laws ER Jr (eds): Brain tumors, ed 2. New York: Churchil Livingstone, 2001, pp 493-524
[21].Zhen L, Yufeng C, Zhenyu S, and Lei X: Multiple extracranial metastases from secondary glioblastoma multiforme: a case report and review of the literature. J Neurooncol 97:451–457, 2010. [Pubmed]
[22].Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, DiPatre PL, et al. Genetic pathways to glioblastoma: a population- based study. Cancer Res 64:6892–6899,2004 [Pubmed]
[23].Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J NeuropatholExpNeurol 64:479–489, 2005. [Pubmed]
[24]Nakamura M, Watanabe T, Klangby U, Asker C, Wiman K, Yonekawa Y, et al. p14 ARF deletion and methylation in genetic pathways to glioblastomas. Brain Pathol 11:159–168, 2001 [Pubmed]


