Research

Adjuvant radiotherapy prevents locoregionalrelapsesinpatientswith early breast cancer staged with sentinel node biopsy
Ricardo Ruano Perez, Manuel Ramos Boyero, Jose Ramon Garcia-Talavera, Juan Ignacio Rodriguez Melcon, Pedro Carreras Soria and Teresa Ramos Grande and Maria Carmen Garcia Macias
NUCLEAR MEDICINE, UNIVERSITY HOSPITAL OF SALAMANCA, , SALAMANCA, Spain
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Aim
The aim of this study is to establish the outcomes of breast cancer patients surgically staged with sentinel lymph node (SLN) biopsy and treated with adjuvant radiotherapy (RT), and to identify possible clinical or tumor-related factors that might help to prevent locoregional relapses.
Method
retrospective study of 317 consecutive breast cancer patients cT1-T2 (<3cm), treated from January 2001 to December 2005 and followed at least for 7 years till 31st December 2012. Events recorded were: axillary lymph node relapse, ipsilateral breast tumor recurrence (BTR), contralateral breast cancer, and distant metastasis (DM). /p>
Results
axillary recurrence occurred in 1 out of 190 SLN negative patients (0.5%). BTR occurred in 16 patients (5.0%), with a median of 20 months from RT. In multivariate analysis HER2+++ status was the only risk factor related to BTR. A BTR related to poor prognosis, as 38% developed metastasis. DM occurred in 7.9% with a median of 2.5 years from surgery. 6 years cancer related survival was 93.4% (297/317) and disease free survival was 88.6% (281/317).
Conclusion
adjuvant radiotherapy, in combination with standard systemic therapy, in early staged breast cancer patients provides excellent long term cure rates with low locoregional relapses. Risk factors identification and surgical stagement with SLN biopsy constitute an optimal approach in breast cancer management.
Introduction
Through the last years the Breast Cancer Units have being incorporating new technological advances to achieve a better characterization and treatment of breast cancer patients. Breast conservative surgery and sentinel lymph node (SLN) stagement have become the two main pillars of the surgical management of early stage breast cancer [1-5]. A complete excision of the tumor followed by an adequate immune histological and hormonal study of the tumor and the SLN achieves an optimal knowledge of tumor biology to set the need or not of adjuvant treatment. Adjuvant radiotherapy is mandatory to consolidate local treatment and to reduce local recurrences in the breast or axilla in patients with conservative surgery with or without SLN metastasis. In a recent systematic review of negative SLN patients, external beam radiation therapy of the breast associated with a significantly lower axillary recurrence rate than non radiated patients, based on the fact that breast or thoracic wall radiotherapy includes radiation of the lower part of the axilla [6].
It is our objective to establish our treatment outcomes of breast cancer patients treated with adjuvant radiotherapy since the performance of the sentinel node technique in 2001, selecting all consecutive patients till December 2005, and establishing the end point follow-up in 31st December 2012. All patients were followed until this date or till death. Also it is our aim to identify possible clinical or tumoral factors that might help to prevent locoregional relapses.
Material and methods
Patient selection
Included all consecutive breast cancer patients cT1-T2 (<3cm) and negative axilla exploration, with surgical staging including sentinel lymph node biopsy and adjuvant radiotherapy between January 2001 and December 2005. All patients gave their written consent for the sentinel node and adjuvant radiotherapy procedures approved by our Hospital. None of the patients was lost in the follow-up.
Sentinel lymph node identification
We employed the combined technique of radiolabelled isotope and blue dye to identify and localise the sentinel lymph node (SLN). In all cases preoperative lymphoscintigraphy was performed, most of them the same day of surgery (68.5% the same day, 31.5% the day before). The way of injection was peritumoral (palpable) or periareolar (non-palpable) with a standard radiopharmaceutical dose of 74-111MBq of 99mTc-colloidal rhenium sulphide (nanocis®). Once the SLN was visualised, either in axilla, internal mammary chain, or both, the SLN projection was marked in the skin.Inside the surgical room, blue dye (lymphazurin®) was injected into the subareolar region about 20 min before the surgery. The SLN was located because of its representative radioactivity with the hand-held gamma probe and/or the blue dye tinction. In these years it was fixed in paraffin, serially sectioned and stained with haematoxylin and eosin (H&E) and immunohistochemistry (IHC) was performed. SLN were classified as negative, isolated tumoral cells (ITC <0.2mm), micrometastasis (<2mm) or macrometastasis (>2mm). Axillary lymph node dissection (ALND) was completed only when the SLN was positive for micro or macrometastasis.
Adjuvant radiotherapy and systemic therapy
For treatment planning, physics calculations were carried out using three dimensional CT simulation in a Somaton scanner (Siemens AG, Munich, Germany). An inclined board (Med-Tec. Inc., Orange City, IA) was used in all cases to minimize CT set up variability. CT was done as in the treatment position with the patient in supine position and the ipsilateral arm in flexion and abduction above the head. Before the CT scan, the radiation oncologist clinically determined the tumorectomy scar and the edge of breast tissues using a radio-opaque skin marker. Whole thorax CT 5 mm slices were obtained and transferred to the planning system with the Plato software (Nucletron, Netherlands) in order to achieve an appropiately delineation of the anatomical structures of interest (skin, affected and non-affected breasts, lung, spinal cord, tumorectomy bed, axillary lymph chain, and supraclavicular fosse lymph chain if indicated). The Planning Target Volume (PTV) was defined as the whole affected breast, delineated from the yugulum to 2 cm bellow the submamary fold, from the anterior midline of the sternum to the lateral breast limit, and from just bellow the skin to the anterior border of the chest wall. The PTV-boost, was defined as the cavity of the tumorectomy with surgical clips with a 1cm security margin in all directions. All patients were treated with high energy photon from a linear accelerator Saturno-42.
Whole breast or thoracic wall RT was delivered to a median dose of 50Gy administered at 1.8-2.0 Gy fractions, five times a week. An electron boost was administered when tumorectomy, delivering in the surgical bed a dose of 10 to 16 Gy at 2 Gy per fraction in 5 to 8 sessions. The possible indications for this boost were: age bellow 50y and/or high risk recurrence cases (surgical margin affected or insufficient, intraductal component >25%, lymphovascular and/or perineural invasion, high nuclear grade, final tumoral size >3cm).
Nodal RT in supraclavicular fosse and internal mammary chain was delivered to a dose of 50Gy at 2Gy fractions in 25 sessions. Nodal RT was not indicated in final pN0. In case of 1 to 3 metastasise nodes after surgery, nodal RT was administered if age was bellow 50y, high nuclear grade, tumoral size greater than 4 cm, insufficient ALND, surgical margin affected, vascular invasion or extracapsular extension. Nodal RT was mandatory if 4 or more lymph node were metastases. In case or mastectomy, thoracic wall RT was delivered to a median dose of 50Gy if surgical margin were affected or more than 4 lymph nodes were metastases. Nodal irradiation criteria were the same as in the conservative surgery patients.Adjuvant systemic therapy was determined and administered by the medical oncology department based on the patients’ risk factors and tumor characteristics (size, axillary involvement, and hormonal receptor status and tumor grade). It included quimiotherapy (QT) and/or hormonal therapy (HT) selected for each individual patient
Follow-up
Follow-up with clinical exploration, tumoral markers, and protocolised imaging techniques (mammography, ultrasounds, bone scan, CT scan or MRI imaging if metastases suspected) was achieved in all 317 patients as it is established in breast cancer follow-up protocol at our institution.
Events recorded were: axillary lymph node relapse, ipsilateral breast tumor recurrence (BTR), contralateral breast cancer, and the evidence of distant metastasis (DM). Global survival, survival related to cancer, and disease-free survival was calculated. End point follow-up was 31stDecember2012. Breast cancers were classified according to TNM 2010 classification of the American Joint Committee on Cancer.
Statistical Analysis
Statistical analysis was done using SPSS 15.0 for windows; p values less than 0.05 were considered significant. Continuous variables were presented as the median and were compared with t-student test. Categorical variables were presented as percentages and the differences compared with chi-square test. Survival analysis was done with a univariate and multivariate Cox regression model based on the relevance of the time when the event happened. Hazard ratios and intervals of confidence were evaluated for the different clinical and pathological variables. In the estimation of the different models the in and out probabilities were 0.05 and 0.10. Cumulative survival after a relapse compared with the group without events was presented with Kaplan Meier curves including the log rank results.
Results
Clinical and pathological characteristics Clinical and pathological characteristics of all consecutive 317 patients and the groups studied in the survival analysis are shown in Table 1. Conservative surgery was pretended in all patients, being possible in 297 (93.7%), and mastectomy was done in 6.3% because of non conclusive margins in the intraoperative analysis of the margins, one third of these with immediate breast reconstruction. SLN histopathology revealed isolated tumoral cells in 2 cases (considered as pN0), and metastasis in 127/317 (39.4%: 102 macro, 25micro). ALND was done in 115 patients, included all macro, and all micro but those where the patient refused second surgery for the ALND or entered a controlled trial.
Table 1 Clinical and pathological characteristics of all patients and groups considered.
|
|
All Patients (317)
|
Non Event (255) A
|
Breast Local Recurrence (12)
|
Metastasis
(20)
|
|
Age (years)
|
|
|
|
|
|
Median
|
57.9
|
58.2
|
54.3
|
53.0
|
|
Range
|
31-89
|
31-89
|
34-75
|
34-75
|
|
Familiar Breast Cancer
|
50 (15.8%)
|
41 (16.1%)
|
2 (16.7%)
|
4 (20.0%)
|
|
Previous Breast Cancer
|
14 (4.4%)
|
---
|
---
|
---
|
|
Palpable Lesion
|
232 (73.2%)
|
193 (75.7%)
|
9 (75.0%)
|
16 (80.0%)
|
|
Non Palpable Lesion
|
85 (26.8%)
|
62 (24.3%)
|
3 (25.0%)
|
4 (20.0%)
|
|
Type of Breast Surgery
|
|
|
|
|
|
Tumorectomy
|
297 (93.7%)
|
244 (95.7%)
|
11 (91.7%)
|
14 (70.0%)
|
|
Mast. + Reconstruction
|
5 (1.6%)
|
2 (0.8%)
|
1 (8.3%)
|
3 (15.0%)
|
|
Mastectomy
|
15 (4.7%)
|
9 (3.5%)
|
0
|
3 (15.0%)
|
|
Isotope Injection
|
|
|
|
|
|
Operation Day
|
217 (68.5%)
|
173 (67.8%)
|
12 (100%)
|
11 (57.9%)
|
|
Day before Operation
|
100 (31.5%)
|
82 (32.2%)
|
0 (0%)
|
8 (42.1%)
|
|
No. SN excised (range)
|
1.65 (1-4)
|
1.68 (1-4)
|
2.0 (1-3)
|
1.75 (1-3)
|
|
Histological Tumor Type
|
|
|
|
|
|
Invasive Ductal
|
269 (84.9%)
|
228 (89.4%)
|
11 (91.7%)
|
18 (90.0%)
|
|
Lobular
|
15 (4.7%)
|
10 (3.9%)
|
1 (8.3%)
|
2 (10.0%)
|
|
In situ
|
14 (4.4%)
|
---
|
---
|
---
|
|
Other
|
19 (6.0%)
|
17 (6.7%)
|
0
|
0
|
|
Receptor Status
|
|
|
|
|
|
Estrogen Receptor +
|
81.0%
|
83.5 %
|
50.0 %*
|
70.0%
|
|
Progestagen
Receptor+
|
75.6%
|
78.0 %
|
50.0%*
|
70.0%
|
|
P53 +++
|
7.0%
|
6.7 %
|
8.3%
|
12.5%
|
|
MIB1 +++
|
11.5%
|
10.2 %
|
16.7%*
|
31.6%*
|
|
HER2 +++ status
|
10.0%
|
8.2 %
|
25.0%*
|
5.3%
|
|
pTumoral size
|
|
|
|
|
|
Tis
|
14
|
---
|
---
|
---
|
|
T1a
|
7
|
7
|
0
|
0
|
|
T1b
|
61
|
52
|
2
|
2
|
|
T1c
|
135
|
117
|
7
|
5
|
|
T2
|
100
|
79
|
3
|
15*
|
|
pNode stage
|
|
|
|
|
|
pN0
|
190 (59.9%)
|
151 (59.2%)
|
7 (58.3%)
|
6 (30.0%)
|
|
pN0mi
|
25 (7.9%)
|
24 (9.4%)
|
1 (8.3%)
|
1 (5.0%)
|
|
pN1a
|
76 (24.0%)
|
65 (25.5%)
|
4 (33.3%)*
|
5 (25.0%)*
|
|
pN2a
|
20 (6.3%)
|
11 (4.3%)
|
0
|
6 (30.0%)*
|
|
pN3a
|
6 (1.9%)
|
4 (1.6%)
|
0
|
2 (10.0%)*
|
|
Final stage
|
|
|
|
|
|
0
|
13 (4.1%)
|
---
|
---
|
---
|
|
IA
|
129 (40.7%)
|
109 (42.7%)
|
5 (41.7%)
|
3 (15.0%)
|
|
IB
|
20 (6.3%)
|
20 (7.8%)
|
0
|
0
|
|
IIA
|
99 (31.2%)
|
86 (33.7%)
|
7 (58.3%)*
|
5 (25.0%)
|
|
IIB
|
31 (9.8%)
|
26 (10.2%)
|
0
|
4 (20.0%)*
|
|
IIIA
|
20 (6.3%)
|
11 (4.3%)
|
0
|
6 (30.0%)*
|
|
IIIC
|
5 (1.6%)
|
3 (1.2%)
|
0
|
2 (10.0%)*
|
a not included the only axillary recurrence nor the 6 contralateral cancers.
* Variable with statistical significance p<0.05 compared with the non-event group (x2).
Systemic Adjuvant Treatment After individually consideration of factors such as age, tumor size, axillary involvement, histological tumor grade, hormonal receptor status, c-erbB2 status and final stagement, patients received added to radiotherapy (RT), hormonal therapy (HT), or chemotherapy (QT). Adjuvant therapy was: only RT in 6 patients (1.6%), RT and HT in 83 (26.2%), RT and QT in 40 (12.6%), and RT with HT and QT in 188 (59.3%). Adjuvant trastuzumab for c-erbB2 positive tumors was not administered because all patients were treated before 2005 when anti-Her2 therapy was not standard yet.
Follow up and events registered
End point of the follow up was 31st, December 2012, all patients were registered until this date or till death. Median follow up of patients without death event was 112 months, with a minimum period of 7 years. Events registered are shown in Table 2:
Table 2 Events occurred through follow-up.
|
|
Events
|
|
Axillary Recurrence
|
1
|
0.5%
|
|
Ipsilateral Breast Cancer Recurrence
|
16
|
5.0%
|
|
Contralateral Breast Cancer
|
6
|
1.9%
|
|
Distant Metastases
|
25
|
7.9%
|
|
Deaths from breast cancer causes
|
20
|
6.3%
|
|
Total Deaths
|
35
|
11.0%
|
Axillary Relapse in 1 patient, that accounts for 0.5% of the 190 SLN- cases. It was a 1.8 cm ductal invasive tumor, treated with tumorectomy, SLN biopsy with 3 negative SLNs, and adjuvant radiotherapy and chemotherapy. Relapse appeared 10 months from surgery, and ALND was done followed by chemotherapy. By the end of follow up is in complete remission.
Cancer in the contralateral breast in 6 patients, who received the appropriate surgical treatment and staging with SLN as with the first cancer, and adjuvant RT and/or QT/HT according to their individual characteristics. By end of follow up none has developed any local or distance relapse. Because of the small number of events and the absence of distant metastases no survival analysis was done.
Breast Tumor Recurrence (BTR)
BTR occurred in 16 patients, treated with surgery (most of them mastectomy), and adjuvant chemotherapy. Median time from surgery was 3.2 years (range 10 months-6.6 years). 6 of them developed distant metastases and died. When compared those BTRs who developed distant metastasis with the BRTs in complete remission after treatment, we did not find any clinical or tumoral factor predictable of metastasis event (statistical analysis exceeded p values >0.05). That is why in survival analysis BTR are all included in the same group.
Distant metastasis
Metastasis appeared in 25 cases (7.9%). 6 of them with previous BTR and 5 with history of previous cancer. Bone was the organ most commonly affected, then liver, lungs and brain. Median time from surgery was 2.8 years. By the end of this follow up 22 had died. The treatment of distant metastasis included systemic chemotherapy, hormonal therapy, surgery, and/or palliative RT.
Univariate and multivariate analysis
To analyze which clinical and biological characteristics of the patients and the tumor might have impact in a local or distant relapse, and so in survival, a univariate and multivariate analysis with Cox regression was performed. For this purpose, we excluded all patients with in situ cancer because of its different natural history compared to an invasive tumor. Also those patients with a previous history of breast cancer were removed as it was not possible to predict which cancer was responsible for the appearance of metastases. Therefore we considered 3 groups: non-event group (255 patients), BTR group (12 patients), and distant metastasis (DM) group (20 patients including those with BTR). Comparing BRT group with the non event group, BRT group patients presented a lower expression of estrogenic tumor receptor (50.0% versus 82.5%, x2, p=0.002), lower expression of progesterone receptor (50.0% versus 78.0%, x2, p=0.006), and amplification or overexpression of HER2+++ (25.0% versus 8.2%, χ2, p=0.016). In the univariate Cox regression analysis these three variables showed hazard ratios with statistical significance, however in the multivariate analysis only the HER2 positive tumor expression presented statistical value (HR=3.00,IC=0.76-11.82,p=0.015) (Table 3). When comparing the DM group with the non event group, we found statistical significance in the univariate Cox regression analysis and afterwards confirmed in the multivariate analysis in the hazard ratios for the following variables: MIB1 overexpression (HR=5.63, IC=1.92-16.67, p=0.002), T2 tumours compared to T1 (HR=4.52, IC=1.47-13.90, p=0.000), pNode metastases (HR=11.03, IC=2.95-41.15, p=0.000), and breast tumour recurrence (HR=157.48, IC=25.72-964.42, p=0.000). See Table 3.
Table 3. Survival analysis.Cox Regression. Univariate and multivariate analysis. Hazard ratios for Breast Local Recurrence and for distant metastases compared with non-event group.
|
|
|
Univariate
|
|
|
Multivariate
|
|
|
|
HR
|
IC 95%
|
p
|
HR
|
IC 95%
|
p
|
|
Local Recurrence Group
|
|
|
|
|
|
|
|
Familiar Breast Cancer
|
1.04
|
0.23-4.75
|
0.959
|
|
|
|
|
Negative Estrogen Receptor
|
4.80
|
1.55-14.90
|
0.007*
|
2.48
|
0.53-11.56
|
0.247
|
|
Negative Progestagen Receptor
|
3.42
|
1.11-10.63
|
0.033*
|
1.59
|
0.36-7.17
|
0.542
|
|
P53 positivity
|
1.49
|
0.19-11.95
|
0.705
|
|
|
|
|
MIB1 positivity
|
1.74
|
0.38-8.07
|
0.477
|
|
|
|
|
HER2 +++ positivity
|
3.77
|
1.00-14.23
|
0.005*
|
3.00
|
0.76-11.82
|
0.015*
|
|
Tumoral grado 3
|
0.30
|
0.04-2.36
|
0.253
|
|
|
|
|
T2 vs T1 tumor
|
0.75
|
0.20-2.78
|
0.669
|
|
|
|
|
pN123 vs pN0
|
1.07
|
0.33-3.58
|
0.902
|
|
|
|
|
|
HR
|
IC 95%
|
p
|
HR
|
IC 95%
|
p
|
|
Metastasis Group
|
|
|
|
|
|
|
|
Familiar Breast Cancer
|
0.60
|
0.14-2.58
|
0.491
|
|
|
|
|
Breast Tumor Relapse
|
23.66
|
7.79-71.85
|
0.000*
|
157.48
|
25.72-964.42
|
0.000*
|
|
Negative Estrogen Receptor
|
2.02
|
0.78-5.29
|
0.148
|
|
|
|
|
Negative Progestagen Receptor
|
1.46
|
0.56-3.81
|
0.437
|
|
|
|
|
P53 positivity
|
1.72
|
0.39-7.57
|
0.473
|
|
|
|
|
MIB1 +++ positivity
|
3.48
|
1.32-9.17
|
0.011*
|
5.63
|
1.92-16.51
|
0.002*
|
|
HER2 +++ positivity
|
0.58
|
0.08-5.29
|
0.600
|
|
|
|
|
T2 vs T1 tumor
|
6.41
|
2.33-17.66
|
0.001*
|
4.52
|
1.47-13.90
|
0.000*
|
|
pN123 vs pN0
|
4.08
|
1.62-10.25
|
0.000*
|
11.03
|
2.95-41.15
|
0.000*
|
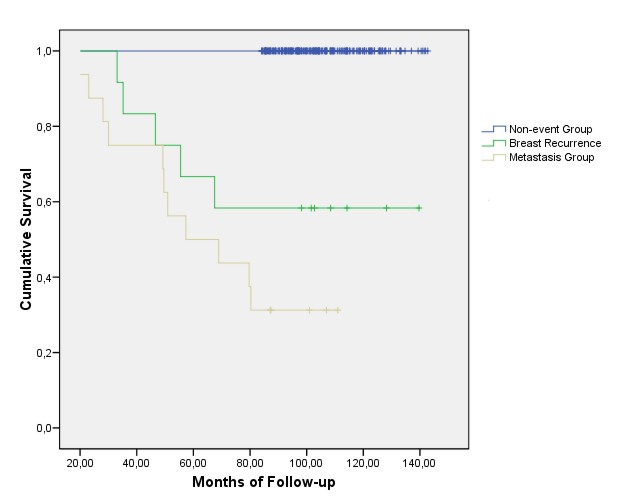
Survival Analysis 7 year overall survival was 89.0% (282/317), cancer related survival was 93.4% (297/317) and disease free survival was 88.6% (281/317).We did not find any survival time difference between patients with axillary relapse and patients without any adverse event (p>0.05).
The appearance of breast recurrence or distant metastasis did imply a significant reduction in survival as the Kaplan-Meier curves obtained for each event clearly show (figure 1).
Fig. 1 Overall Survival according to events detected
Discussion
With more than 10 years of experience in the performance of the sentinel lymph node (SLN) biopsy at the Breast Cancer Unit of the University Hospital of Salamanca, with over 1200 procedures performed, in this work we present our results, with a long follow-up of 7 years, of all consecutive early staged breast cancer patients where the surgical staging included the SLN procedure and received adjuvant breast radiotherapy. SLN biopsy indication came after a negative clinical and ultrasound (in T2 tumors) axilla in all cases. SLN histological analysis was in all cases with H&E and IHC, however nowadays the OSNA method based on the CK19 mRNA copy number amplified in SLN lysates is the method used. We decided not to exclude patients with mastectomy as the original surgical planning included conservative surgery, however it must be mentioned that radiotherapy indication came after more than 4 axillary nodes affected, and that explains why these patients where to a higher risk to develop distant metastasis. It is also important to point out that the sentinel node procedure was indicated in high risk in situ patients, that is why in situ breast cancers were included in the study.
Our false negative rate for the SLN technique was 0.5%, an optimal and desirable rate similar to what has being published in a recent review of patients treated with adjuvant radiotherapy [6]. This value is lower to the 1-3% expectable rate if we had also included patients without adjuvant radiotherapy, as breast radiotherapy indirectly includes in the tangential fields the lower part of the axilla favouring a lower axillary relapse [7-8]. The radiotherapy protocol of the Radiotherapy Department, following international guidelines, includes in case of conservative surgery an electron boost to the tumor bed [9-12]. Despite this treatment, 5% patients developed a breast tumoral recurrence (BTR), value, although undesirable, in the range of 3-6% published by other groups [13-16]. We found in the multivariate analysis that HER2 status was the only factor associated to a BTR. This fact correlates with Ngyuyen et al. [17], who found a higher rate of BTR in HER2 positive patients, especially when added negative estrogenic and progesterone receptors. However, it must be considered that this study has only included patients in the previous years to the addition of adjuvant trastuzumab to systemic treatment early breast cancer. Other groups referred to a low age under 40 years or the presence of lymphovascular invasion as other risk factors, but in our results these variables became not significant [18].
We did not find any clinical or histological variable able to predict which patients with BTR would be in risk of developing distant metastases. However, in our series, after a BTR, and despite surgical and adjuvant therapy, 38% patients developed distant metastases and as a consequence had influence in survival. This result agrees with Anderson et al [19] and de Boch et al [20], who after following 3799 and 3601 patients respectively, considered the existence of a BTR as risk factor for latter distant metastasis, and therefore BTR patients should be more strictly studied and followed.
We also analyzed variables related to distant metastases, being statistically significant the mib1 overexpression, T2 pathological size tumors, and also lymph node metastases. Also, as mentioned, BTR was a determined factor for the development of metastasis disease. These results must be interpreted with caution due to the limited number of patients with metastasis, as our study only includes early staged breast cancer. Of course it must be mentioned the relevance of node stagement as it has impact in patient´s survival, and the relevance of the sentinel node biopsy as the method of choice for axillary stagement of breast cancer patients.
Our results in overall cancer survival and disease free survival of 93.4% and 88.6% are optimal and similar to the prospective randomized trial NSABP B-32
Conclusion
Adjuvant radiotherapy, in combination with standard systemic therapy, in early staged breast cancer patients provides excellent long term cure rates with low locoregional relapses. Risk factors identification and surgical stagement with SLN biopsy constitute an optimal approach in breast cancer management.
Acknowledgements
We are thankful to all the personal staff of the Nuclear Medicine, Surgery, Radiation Oncology, Oncology, and Pathology Departments of the University Hospital of Salamanca.
Conflicts of Interest
The authors declare that there are no conflict of interests.
Authors’ Contributions
RRP: Preparation of the manuscript, editing of the final version.
MRB: Literature search and preparation of manuscript
JRGT: Preparation of manuscript
JIRM: Preparation of manuscript
PCS: Preparation of manuscript
TRG: Preparation of manuscript
MCGM: Preparation of manuscript
Funding
None
References
[1]Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, Ashikaga T, Weaver DL, Mamounas EP, Jalovec LM, Frazier TG, Noyes RD, Robidoux A, Scarth HM, Wolmark N. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial.Lancet Oncol. 2010; 11:927-33.[pubmed]
[2].Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010; 252:426-32.[pubmed]
[3].Hunt KK, Ballman KV, McCall LM, Boughey JC, Mittendorf EA, Cox CE, Whitworth PW, Beitsch PD, Leitch AM, Buchholz TA, Morrow MA, Giuliano AE. Factors associated with local-regional recurrence after a negative sentinel node dissection: results of the ACOSOG Z0010 trial. Ann Surg. 2012; 256:428-36.[pubmed]
[4].Veronesi U, Viale G, Paganelli G, Zurrida S, Luini A, Galimberti V et al. Sentinel lymph node biopsy in breast cancer. Ten Years results of a randomized controlled study. Ann Surg 2010; 251:595-600. [pubmed]
[5].Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011; 305:569-75.[pubmed]
[6].Van Wely BJ, Teerenstra S, Schinagl DA, Aufenacker TJ, de Wilt JH, Strobbe LJ. Systematic review of the effect of external beam radiation therapy to the breast on axillary recurrence after negative sentinel lymph node biopsy. Br J Surg. 2011; 98: 326-33.[pubmed]
[7].Wernicke AG, Goodman RL, Turner BC, Komarnicky LT, Curran WJ, Christos PJ, Khan I, Vandris K, Parashar B, Nori D, Chao KS. A10-year follow-up of treatment outcomes in patients with early stage breast cancer and clinically negative axillary nodes treated with tangential breast irradiation following sentinel lymph node dissection or axillary clearance. Breast Cancer Res Treat. 2011; 125: 893-902.[pubmed]
[8].van Wely BJ, van den Wildenberg FJ, Gobardhan P, van Dalen T, Borel Rinkes IH, Theunissen EB, Wijsman JH, Ernst M, van der Pol CC, Madsen EV, Vles WJ, Wauters CA, de Wilt JH, Strobbe LJ. Axillary recurrences after sentinel lymph node biopsy: a multicentre analysis and follow-up of sentinel lymph node negative breast cancer patients. Eur J Surg Oncol. 2012; 38:925-31.[pubmed]
[9].Jones HA, Antonini N, Hart AA, Peterse JL, Horiot JC, Collin F, Poortmans PM, Oei SB, Collette L, Struikmans H, Van den Bogaert WF, Fourquet A, Jager JJ, Schinagl DA, Wárlám-Rodenhuis CC, Bartelink H. Impact of pathological characteristics on local relapse after breast-conserving therapy: a subgroup analysis of the EORTC boost versus no boost trial. J Clin Oncol. 2009; 27:4939-47.[pubmed]
[10].Poortmans PM, Collette L, Bartelink H, Struikmans H, Van den Bogaert WF, Fourquet A, Jager JJ, Hoogenraad W, Müller RP, Dubois JB, Bolla M, Van Der Hulst M, Wárlám-Rodenhuis CC, Pierart M, Horiot JC; EORTC Radiation Oncology and Breast Cancer Groups. The addition of a boost dose on the primary tumour bed after lumpectomy in breast conserving treatment for breast cancer. A summary of the results of EORTC 22881-10882 "boost versus no boost" trial. Cancer Radiother. 2008; 12:565-70.[pubmed]
[11].Livi L, Borghesi S, Saieva C, Fambrini M, Iannalfi A, Greto D, Paiar F, Scoccianti S, Simontacchi G, Bianchi S, Cataliotti L, Biti G. Benefit of radiation boost after whole-breast radiotherapy. Int J Radiat Oncol Biol Phys. 2009; 75:1029-34.[pubmed]
[12].López Carrizosa MC, Samper Ots PM, Vallejo Ocaña C, Rodríguez Pérez A, Sáez Garrido Jde D, Delgado Pérez JM. Early stage breast cancer conserving treatment: high dose rate brachytherapy boost to the tumour bed. Clin Transl Oncol. 2005; 7:344-50.[pubmed]
[13].Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011; 378:1707-16.[pubmed]
[14].Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H, Perera F, Fyles A, Schneider K, Gulavita S, Freeman C. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010; 362:513-20.[pubmed]
[15].Sanpaolo P, Barbieri V, Genovesi D. () Prognostic value of breast cancer subtypes on breast cancer specific survival, distant metastases and local relapse rates in conservatively managed early stage breast cancer: a retrospective clinical study. Eur J Surg Oncol. 2011; 37:876-82.[pubmed]
16].Arvold ND, Taghian AG, Niemierko A, Abi Raad RF, Sreedhara M, Nguyen PL, Bellon JR, Wong JS, Smith BL, Harris JR. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011; 29:3885-91.[pubmed]
[17].Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, Bellon JR, Wong JS, Smith BL, Harris JR. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008; 26:2373-8.[pubmed]
[18].Algara López M, Sanz Latiesas X, Foro Arnalot P, Lacruz Bassols M, Reig Castillejo A, Quera Jordana J, Membrive Conejo I, Lozano Galán J, Rodríguez de Dios N. () Risk factors of local relapse in breast cancer: the importance of age. Clin Transl Oncol. 2007; 9:110-6.[pubmed]
[19].Anderson SJ, Wapnir I, Dignam JJ, Fisher B, Mamounas EP, Jeong JH, Geyer CE Jr, Wickerham DL, Costantino JP, Wolmark N. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009; 27:2466-73.[pubmed]
[20].De Bock GH, Putter H, Bonnema J, van der Hage JA, Bartelink H, van de Velde CJ. The impact of loco-regional recurrences on metastatic progression in early-stage breast cancer: a multistate model. Breast Cancer Res Treat. 2009; 117:401-8.[pubmed]


