Original Article

Myelodysplastic Syndrome (MDS) with Isolated 5q Deletion ((5q –Syndrome): Report of Two Cases with Review of Literature
1 Mona Bargotya, 1 Ankita Mehta 2 Sarjana Dutt 2 Tejinder Singh33Harsh Dua,
- 1Rajiv Gandhi Super Speciality Hospital,
- 2Oncquest Laboratories Ltd,
- 3Apollo Hospital, New Delhi, India.
- Submitted:Monday, April 30, 2018
- Accepted: Thursday, May 3, 2018
- Published: Thursday, May 3, 2018
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Cytogenetics is frequently seen in myelodysplasia and deletion of the long arm of chromosome 5, del (5q) is the most frequently observed cytogenetic abnormality in patients with myelodysplastic syndrome (MDS). This distinct hematologic phenotype with5q- deletion as the solekaryotypic abnormality is termed as 5q- syndrome. The World Health Organisation in 2001 recognized MDS with isolated del (5q) as a unique, narrowly defined distinct entity. The 5q-syndrome is the most distinct of all the myelodysplastic syndromes, and the molecular basis of this disorder still remains unknown. Herein, we present two cases of 5q-syndrome. Both the cases were elderly males who presented with refractory anemia. Bone marrow aspirate smears and bone marrow biopsy showed morphological features suggestive of 5q-syndrome FISH was performed to confirm 5q syndrome. Advances in the understanding of the pathogenesis of 5q deletion syndrome have suggested that it is actually a highly heterogeneous disease with variable prognosis. We discuss clinical and morphological features as well as new evolving therapies for reversal of anaemia and treatment of 5q syndrome.
Key words:
Myelodysplastic syndrome, Lenalidomide, 5q deletion
Introduction
Myelodysplastic syndromes (MDS) are aheterogeneous group of clonal hematopoietic stem cell disorders characterized by ineffective hematopoiesis leading to peripheral blood cytopenias, hypercellular bone marrow,dysplasia in one or more of the major myeloid cell lines and by increased incidence of progression to acute myeloid leukemia (AML). Cytopenias occur due to presence of enhanced degree of apoptosis. Cytogenetic abnormalities are commonly encountered in MDS, seen in approximately 80% of secondary MDS cases (therapy related) and around 50% of the de novo cases of MDS [1].One of the most common cytogenetic abnormality observed in de novo cases is isolated deletion of long arm of chromosome 5 i.e del(5q) which is present in about 15% of cases, seen as an isolated abnormality or with additional karyotypicanomalies.The World Health Organization classification in 2001 recognizes MDS associated with del (5q) – the 5q-syndrome, as a distinct entity, defined as MDS with less than 5% bone marrow blastsin addition to del (5q) as the sole karyotypic abnormality [2]. It is characterized by refractory anemia with characteristic clinical features; female predominance (unlike other MDS), macrocytosis, erythroid hypoplasia, frequent thrombocytosis, hypolobatedmicromegakaryocytes with a slow rate of progression to acute myeloid leukemia in comparison to other types of MDS. With the supportive therapy, the prognosis of 5-q syndrome is favourable.5q deletion is also observed in AML, with important prognostic significance.Prognosis of AML with 5q- deletion is generally unfavourable and is associated with rapid disease progression, poor outcome and survival, especially if associated with complex karyotype [3].
Case Reports
Herein, we present 2 cases of 5q -syndrome. Peripheral smear, bone marrow aspiration and biopsy were done for both these cases.
Case I: 79 years old male presented with refractory anemia. Complete blood count revealed hemoglobin of 2 gm/dl and peripheral smear showed predominantly normocytes along with many microcytic hypochromic cells, few target cells and tear drop cells. Platelet count was increased on smear. Bone marrow examination was hypercellular showing erythoid hyperplasia with macroblastic maturation. Myelopoiesis showed normal maturation. Megakaryocytic hyperplasia was present and themegakaryocytes were small, round to oval, unilobated or hypolobated (Figure 1A). Bone marrow biopsy was mildly hypercellular with presence of many dwarf megakaryocytes(Figure 1C).
Although commonly we observe macrocytic anemia in MDS 5 q deletion patients but in our case we observed patient had microcytic hypochromic anemia with MCV of 65.3 fL, MCH 19.5pg and MCHC 29.8 g/dl. Further the iron profile of the patient was performed and patient was found to be iron deficient with low serum iron and ferritin levels.
recurrent anemia. Complete blood count revealed hemoglobin of 4gm/dl peripheral smear showed predominantlymacrocytes along with many normocytic normochromic cells, occasional macro-ovalocytes, tear drop cells and spherocytes. Platelets were adequate. Bone marrow examination was normocellular with erythoid hyperplasia with macronormoblastic maturation. Myelopoiesis showed normal maturation with megakaryocytic hyperplasia. Hypolobatedmicromegakarocytes were present. (Figure 1B) Bone marrow biopsy was normocellular with abundant megakaryocytes. (Figure 1D) In both the cases bone marrow aspiration and biopsy features were suggestive of MDS.Fluorescent in situ hybridization (FISH) (Figure 2) and cytogenetic analysis (Figure 3) was performed on bone marrow samples. Fluorescent signals were visualized with fluorescence microscope equipped with double band-pass filter. Chromosome identification and karyotyping was done according to International System for Human Cytogenetic Nomenclature.

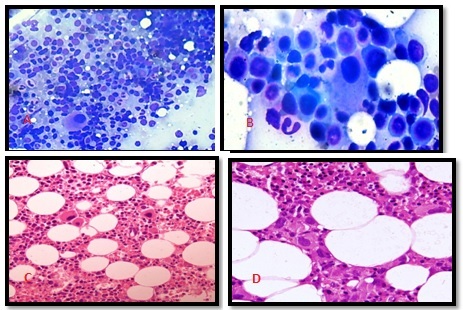
Figure1 (A): (40X), Bone marrow aspirate smear showing myeloid proliferation with normal maturation and megakaryocytic hyperplasia. (B):(100X), Bone marrow aspirate smear showing characteristic hypolobated dwarf megakaryocytes. (C):(10X), Bone marrow trephine biopsy showing myelopoiesis with normal maturation and megakaryocytic hyperplasia. (D):(40X),Bone marrow trephine biopsy section multiple unilobated dwarf megakaryocytes.

Figure 2: (FISH): Interphase FISH showing 2 green signals and 1 red signal suggesting loss of 5q.

Figure 3: Karyotyping showing 5q deletion
5q deletion syndrome was confirmed in both the cases by fluorescent in situ hybridization (FISH) as well as karyotyping. In the present study both the patients were treated with immunomodulatory drug Lenalidomide. Initially they were started on the dose of 10mg/day for 21 days every 4 weeks. After 5 cycles of this treatment the haematological profile of the patient was assessed and it was observed that both the patients responded well to the treatment. The patient was then put on the tapering dose of Lenalidomide. Both the patients are on regular follow up and they not only showed good response to the drug but also marked reduction in the blood transfusion requirement was observed.
Discussion
Myelodysplastic syndromes are clonal stem cell disorders characteristically seen in elderly females with a median age group of 70 years; whereas therapy related MDS show male predominance [4] .Chromosomal deletions and monosomies are frequently seen in MDS. Most of the malignant diseases of myeloid lineage are characterized by single chromosomal abnormalities like translocation; however MDS is typically associated with cytogenetic deletions like 5q, 7q, 20q and 12p [5].
Most common cytogenetic abnormality seen in MDS is deletion of chromosome 5, which is defined as interstitial deletion of long arm of chromosome 5(del5q) or complete loss of entire chromosome 5. MDS with acquired deletion of long arm of chromosome 5(del5q) was reported by Herman van den Berghe et al in 1974 [6]. This distinct entity was further described in detail by Sokal, Van den Berghe and co-workers in 1975 [7].In 2001, World Health Organization published a new classification for hematopoietic and lymphoid neoplasms which recognized MDS with isolated deletion 5q, the 5q syndrome, as a unique narrowly defined entity.In this type of MDS, the 5th chromosome is missing or has been deleted [8].Additional cytogenetic abnormalities and presence of ≥ 5% blasts in the blood or marrow, excludes the diagnosis of 5q- syndrome.
According to the recent WHO 2016 classification of MDS; isolated del(5q) should be diagnosed even if there is 1 additional cytogenetic abnormality along with del(5q) with an exception of presence of monosomy 7 or del(7q). It states that there is no adverse effect even if 1 additional chromosomal abnormality present with MDS [9]. The 5q syndrome originates in human hematopoietic stem cell and it is seen mainly in females of advanced age group. The region for 5q syndrome is located at 5q32, whereas the other aggressive forms of MDS and AML are located at band 5q31. However, a distinct region at 5q31 is frequently found to be deleted in patients who have deletion 5q associated with other chromosomal abnormalities or who have previously undergone chemotherapy [10]
In general, patients with myelodysplastic syndrome (MDS) typically present with features associated with cytopenia, such as fatigue, easy bleeding or bruising, or infection. MDS with isolated 5q deletion cases typically present with persistent anemia that has no other identifiable cause. It can present as refractory anaemia, neutropenia or thrombocytopenia. However, the most common cytopenia in patients with 5q syndrome is anemia. They present with a striking macrocytic anemia and are generally transfusion-dependent. Haploinsufficiency of the ribosomal gene RPS14 is known to cause the erythroid defect in the 5q- syndrome and leads to anemia via both p53-dependent and p53-independent tumor suppressor effects, p21 and cell cycle arrest [11]. Platelet count is usually normal or may show thrombocytosis in the peripheral blood. Leukocytopenia may be present; however, the number of granulocytes is typically normal. Bone marrow aspirates show predominantly megakaryocytic dysplasia with hypolobated micromegakaryocytes. Deletion of MicroRNA genesi.e miR-145 and miR-146ais associated with thrombocytosis and megakaryocytic dysplasia observed in 5q syndrome [12].The bone marrow biopsy in these cases is usuallynormocellular or mildly hypercellular with presence of numerous micromegakaryocytes. This is in concordance with the findings of peripheral blood and bone marrow in the present study. Some of the other genes which are involved in 5q syndrome include tumor suppressor EGR1, CTNNA1, and CDC25C and SPARC. This tumor suppressor gene SPARC (Secreted protein acidic and cysteine rich) has anti-proliferative, anti-adhesive and anti-agniogeneic effect [13].
According to International Prognostic Scoring System (IPSS) MDS is classified into 3 low risk, intermediate risk-1 and intermediate risk-2 or high risk group. With the help of IPSS system prognosis of disease and likelihood of transformation into AML can be predicted. The scoring of IPSS system is based on bone marrow immature cell count (blast count), number of blood cells affected and number and type of genetic changes. According to IPSS, MDS 5 q deletion syndrome is classified as low risk group [15].
In many people, it can remain stable for many years, however transformation into AML is rare in this syndrome. However, the co-existence of 5 q deletion with other karyotypic abnormalities and patients with secondary MDS show rapid transformation to AML. In the study done by Giagounidis, they showed that in MDS patients with deletion 5q in association with excess blasts and those with an additional chromosomal abnormality have a significantly shorter survival than patients with isolated deletion 5q [14]. So, the presence of chromosome 5 q deletion is not only an important prognostic factor in the management of MDS patients but it is also used as clonal marker of the disease. Usually isolated 5 q deletion syndrome has good prognosis but most of the patients of MDS 5q deletion over the course of disease become transfusion dependent which increases morbidity and decreases the quality of life. Therefore, the main aim of management is early detection of MDS in order to keep the patient independent of transfusion.
Usually MDS responds poorly to erythropoiesis stimulating agent, but now with the advent of immunomodulatory drug Lenalidomidethere has been dramatic improvement in patients with the 5q- syndrome. It is used for the treatment of transfusion dependent lower risk group of isolated 5q syndrome. Lenalidomide up regulates the expression of several genes like SPARC, TGF-B. It also promotes degradation of p53 by inhibiting auto-ubiquitination of MDM2 in 5 q deletion syndrome [15]. In patients with del(5q) TP53 mutation predicts poorer response to lenalidomide, so its evaluation in patients with MDS with isolated del(5q) to help identify an adverse prognostic subgroup [16].
Conclusion
The 5q- syndrome is the most distinct of myelodysplastic syndromes and the most striking morphological abnormality in 5q deletion concerns the megakaryocytes, especially their nuclei which are generally small, round or oval and hypolobated or unilobated. Extensive investigations have been done on the molecular basis of this disorder. Great progress made in understanding the pathogenesis of the disease, has not only helped to explain the clinical findings but also directed to future therapy. Although del(5q) is common in MDS, it may not present in all cells, thereby leading to diagnostic challenges.
Although the disease can be diagnosed by conventional cytogenetic analysis (CCA) but FISH is more sensitive than CCA and it allows for the study of non-dividing cells, detection of minor abnormal clones and submicroscopic lesions. So, FISH improves the detection of deletion 5q31-q32 in patients of MDS without cytogenetic evidence of deletion 5q. As patients of primary MDS with isolated 5q deletion syndrome are very well managed with immunomodulatory drug lenalidomide and it is associated with good prognosis, therefore early and proper detection of this chromosomal abnormality is of prime importance.
Conflict of Interests
The authors have no conflict of interest.
References
[1].Bernasconi P, Klersy C, Boni M, et al. Incidence and prognostic significance of karyotype abnormalities in de novo primary myelodysplastic syndromes: a study on 331 patients from a single institution. Leukemia 2005; 19: 1424-31.[PubMed]
[2].Jadersten M. Pathophysiology and treatment of the myelodysplastic syndrome with isolated 5q deletion. Hematologica 2010;95(3)348-51.[PubMed] [PMC Full Text]
[3].Charrin C. del(5q) in myeloid neoplasms. Atlas of Genetics and Cytogenetics in Oncology and Hematology1998;2(3)88-90 http://atlasgeneticsoncology.org/Anomalies/del5qID1092.html
[4].Pellagatti A, Boultwood J. Recent advances in the 5q- Syndrome. Mediterr J Hematol Infect Dis 2015;7(1).[PubMed] [PMC Full Text]
[5].Greenberg, P., Cox, C., Le Beau, M.M., Fenaux, P., Morel, P., et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997;89: 2079- 88.[PubMed] [Free Full Text]
[6].Van Den Berghe H., Cassiman, J.J., David, G., Fryns, J.P., Michaux, J.L. &Sokal, G. Distinct hematological disorder with deletion of long arm of no. 5 Chromosome. Nature 1974; 251: 437-438.[PubMed]
[7].Sokal G, Michaux JL, Van Den Berghe, H, Cordier A, Rodhian J, Ferrant A, et al. A new haematologic syndrome with with a distinct karyotype: the 5 q chromosome. Blood 1975;46:519-33.[PubMed] [Free Full Text]
[8].WHO Classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. Lyon: IARC; 2008. 88-90 p.
[9].Daniel A. Arber, Attilio O, Robert H, Jurgen T, Michael J. Borowitz, Michelle M. Le Beau et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127(20): :2391-2405.[PubMed] [Free Full Text]
[10].Mohamedali A, Mufti GJ. Van-den Berghe's 5q- syndrome in 2008. Br J Haematol. 2009. 144(2):157-68.[Pubmed]
[11].Nakhoul H, Ke J, Zhou X, et al. Ribosomopathies: Mechanisms of disease. Clinical Medicine Insights: Blood Disorders 2014;7: 7–16.[PubMed] [PMC Full Text]
[12].Starczynowski DT; Kuchenbauer F; Argiropoulos B; et al. "Identification of miR-145 and miR-146a as mediators of the 5q- syndrome phenotype". Nature Medicine 2010;16(1): 49–58.[PubMed]
[13].Komrokji RS, List AF. Role of lenalidomide in the treatment of myelodysplastic syndromes. SeminOncol 2011; 38: 648-57.{PubMed]
[14].Giagounidis AA, Germing U, Haase S, Hildebrandt B, Schlegelberger B,Schoch C, et al.Clinical, morphological, cytogenetic, and prognostic features of patients with myelodysplastic syndromes and del(5q) including band q31. Leukemia 2004; 18:113–9.[PubMed]
[15].Wei S, Chen X, McGraw K, et al. Lenalidomide promotes p53 degradation by inhibiting MDM2 auto-ubiquitination in myelodysplastic syndrome with chromosome 5q deletion. Oncogene 2013; 32: 1110-20.[PubMed] [PMC Full Text]
[16].Mallo M, Del Rey M, Ibanez M, et al. Response to lenalidomide in myelodysplastic syndromes with del(5q): influence of cytogenetics and mutations. Br J Haematol. 2013; 162(1):74-86[PubMed]
.

