Original Article

Molecular Analysis of X-Linked Inhibitor of Apoptotic Protein (XIAP) and Caspase -3 in Oral Cancer
1Manisha Shrivastava, 1Rashmi Bathri,
1Shweta Mishra, 3Pallavi Joshi, 3Anupriya Rathore,
2,4Manoj Pandey
1Departments of Transfusion
Medicine and 2Surgical Oncology, Bhopal Memorial Hospital and Research Center, Bhopal, India
3Department of Biotechnology, IEHE, Bhopal, India
4Department of Surgical Oncology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Sunday, March 19, 2017
Thursday, April 20, 2017
Saturday, April 22, 2017
Abstract
Introduction
Over-expression of anti-apoptotic proteins such as X-linked inhibitor of apoptotic protein (XIAP) leads to defects in caspase-3 mediated apoptosis regulation and is one of the main causes of cancer development and is associated with poor clinical outcome. Targeting of XIAP in conventional cancer therapy has the potency to significantly improve success rates of cancer treatment and the survival of patients. Our study is a preliminary effort to study the gene expression of less studied XIAP and caspase -3 in oral cancers by amplification of these genes through PCR method and 8-oxodG, a marker of oxidative damage.
Material and Methods
This Prospective pilot study is based upon the expression of XIAP and caspase protein among patients with oral cancer. PCR- gel electrophoresis was performed to analyze the gene expression and level 8-oxo dGwas measured by performing ELISA.
Results
Out of the 11 samples, 7 pure DNA samples showed amplification of caspase-3 and β-Actin gene as internal control. The median value of 8-oxodG was 9.47 nM (interquartile range: 5.64–41.13 nmol).
Conclusions
We have optimized the protocol for the molecular analysis of these genes through PCR and propose to continue the study on a larger sample size with suitable controls and also include real time PCR and Western blot techniques for evaluating the expression of XIAP protein and caspase -3 and its role in future target therapy in oral cancer patients.
Keywords
Apoptosis; oral cancer; XIAP; caspase-3; oxidative stress.
Introduction
India is a contributor by 7.8% to the global burden of cancer and 8.33% of global cancer deaths occur in India [1]. World-wide, the head and neck cancers form the sixth most common cancer [2]. Oral cancer or mouth cancer is a subtype of head and neck cancer and the eleventh most common malignancy in the world, with squamous cell carcinoma being a predominant histological type. Oral cancer is the 3 rd most common type of cancer and the most common cancer in males which accounts for over 30% of all cancers in India. Misconceptions, lack of awareness, exposure to tobacco, lower socio-economic status, lack of diagnostic, treatment facilities, uniform protocols for management are some reasons the disease burden increased over the years [3,
4]. However, research and development has also lead to better understanding of molecular biology of cancers and newer therapies and early interventions have created hope for better survival of patients. Over-expression of anti-apoptotic proteins such as inhibitor of apoptosis proteins (IAPs) leads to defects in apoptosis (cell death) regulation and is the main cause of cancer development, as a result of malfunction and interference with apoptotic signaling via death receptors or intrinsic cell death pathways. IAPs, bind caspases as they have one to three common structures, the baculovirus-IAP-repeat (BIR)-domains. Out of the 8 human IAPs, X-linked inhibitor of apoptosis protein (XIAP) is the most potent and best-defined anti-apoptotic IAP family member. XIAP selectively binds and inhibits caspases 3, 7 and 9, but not caspase-8. Thus, XIAP is an attractive target for novel therapeutic agents for the treatment of malignancy like the Antisense oligonucleotides directed against XIAP being evaluated in clinical trials and small molecule XIAP inhibitors in preclinical development [5]. Furthermore increased XIAP levels have also been reported for ovarian carcinoma [6], B-cell Non-Hodgkin and Hodgkin lymphoma [7] clear cell renal cancer [8,
9], esophageal carcinoma [10], and non-small cell lung cancer [11].
Targeting of IAPs in conventional cancer therapy has the potency to significantly improve success rates of cancer treatment and the survival of patients. As evident in various studies, XIAP is found over-expressed in many cancer tissues, it could be significant biomarker and target for cancer therapy, but this could not be generalized for all cancer tissues and its expression may not be always correlated with adverse clinical outcome. Thus it becomes important to identify patient subgroups, through additional clinical studies, which harbor the possibility to benefit from XIAP-targeted therapy. Especially in oral cancer, a major cause of morbidity and mortality due to cancer, where there is paucity of studies, XIAP in combination with other biomarkers may evolve as a predictive tool and as a drug target in personalized cancer therapy. The caspases are crucial mediators of programmed cell death (apoptosis). Among all, Caspase-3 is a frequently activated death protease, catalyzing the specific cleavage of many key cellular proteins. Caspase - 3 are involved in two major pathways in apoptosis [12].
Unlike survivin which has been widely studied expression of XIAP and caspase- 3 in oral cancer has not been studied. The overexpression of cytoplasmic survivin in oral cancers and premalignant lesions has been shown to occur suggesting that survivin has a role in suppression of apoptosis at early and late stages of oral carcinogenesis. The increased expression of caspase -3 and nuclear survivin also indicate that caspase - 3 promotes apoptosis and cell proliferation is increased due to survivin in late stages [13]. 8-oxodG, is a major oxidative DNA-damage product that can produce mutation. Oxidative stress may play a role in tumor progression and increase in 8-oxodG levels in DNA of squamous cell carcinoma of head and neck patients has been observed [14]. The aim of this study was to find the XIAP and Caspase-3 gene amplification in oral cancer patients by Polymerase Chain Reaction (PCR) and electrophoresis. The biomarker 8-oxodG was also studied for quantification of endogenous oxidative DNA damage and as a factor of initiation and promotion of carcinogenesis in oral cancer patients.
Patients and Methods
The study was approved by the Institutional ethical committee (IEC). The study group consisted of 11 patients, diagnosed with oral cancer visiting the outpatient department of Surgical Oncology and undergoing biopsy procedure or surgery. Approximately 5 mm tissue sample was collected from those patients who were willingly to participate in this study and informed consent was taken as approved by IEC. Samples were collected in normal saline and stored at minus 80°C till further use. Urine sample (3 ml) from these patients was also collected in sterile urine containers for the analysis of oxidative damage.
DNA Isolation and Primer designing: DNA isolation was done by phenol chloroform method. Purity and concentration of DNA was determined by spectrophotometric analysis at A260/280nm. Quantification and purity of DNA: Obtained DNA was quantified using spectrophotometer; 15µl of DNA was diluted with 1485 µl of distilled water in an quartz cuvette for the quantification of DNA. Three consecutive readings of each sample were obtained and mean was calculated. Concentration of DNA was calculated using the formula: Optical density × dilution factor × conversion factor Where, dilution factor is 100 i.e. DNA was 100 times diluted & conversion factor is 50 ng /µl (standard for ds DNA). The scoring pattern was used to discriminate sex (Male=1, Female=2), age group (≤25=1, 26-50=2,50-75=3, ≥75=4) and DNA purity (1.8-2.2=1,others=2) of the studied samples.
Specific primer designing was done by using NCBI Primer Blast considering all relevant factors.
XIAP
Forward, 5′-CAGTATCCAGCTGCCACTGAG -3′
Reverse: 5′-TGGGTGAATTGTCAACTGGGAG -3′
Caspase 3
Forward: 5′-TTCAGAGGGGATCGTTGTAGAAGTC-3′
Reverse: 5′-CAAGCTTGTCGGCATACTGTTTCAG-3′
β –Actin
Forward: 5′ AGTTGCGTTACACCCTTTCTTG-3′
Reverse: 5′ TGCTGTCACCTTCA CCGTTC-3′
Polymerase Chain Reaction (PCR): PCR on thermal cycler (PTC-200, MJ Research, Biorad, US) was done for molecular detection of XIAP and caspase 3 gene using primers to amplify fragments of 480bp for XIAP and 240bp, for caspase-3 and 564bp for β -Actin (internal control). The PCR programme is given in
Table 1.
|
Step
|
Step
|
Temperature
|
Time
|
|
1
|
Pre Denaturation
|
94ºC
|
5'
|
|
2
|
Denaturation
|
94ºC
|
2'
|
|
3
|
Annealing
|
55ºC
|
30 sec
|
|
4
|
Extension
|
72ºC
|
2'
|
|
5
|
Go to 2 , 35 times
|
|
|
|
6
|
Final extension
|
72ºC
|
7'
|
Gel electrophoresis and analysis: 2% agarose gel was prepared by dissolving the agarose in 1x Tris-Acetate- EDTA (TAE) buffer. The agarose was dissolved in the buffer by heating, melted agarose was allowed to cool and 5µl ethidium bromide dye was added when agarose was cooled up to 75°C this solution was dispended in a gel mold fitted appropriate well forming comb after cooling comb was removed carefully. PCR products were run at 2% agarose gel with 1 kb marker at 70 Volts for 30 minutes. Gel pictures were captured by gel documentation unit (Vilber Lourmat) Gel Analysis was performed by My Image Analysis Software v 1.1 (Thermofisher).
Quantification of 8-oxodG by ELISA: Elisa (Trevigen, USA) was performed with the urine samples; absorbance was taken at 450 nm. 8-oxodG standard curve was plotted to determine sample concentrations using the calculations as per worksheet provided on the website.
Results
PCR and electrophoresis analysis of XIAP and caspase 3 gene region was applied to patient samples with reference to the housekeeping gene β -Actin.
Table 2 shows the patient information (age, sex, risk factors) and purity and concentration of isolated DNA. Out of 11 DNA samples, 7 were score 1 while 4 DNA samples were score 2 (Figure 1).
Table 2. Patient information and purity and concentration
of isolated DNA.
|
Patient ID
|
Sex
|
Age group
|
Oral Cancer Risk Factors
|
Purity
|
Concentration ng/µl
|
|
1
|
1
|
3
|
Alcohol, tobacco, smoking
|
2
|
27.5 ng/µl
|
|
2
|
1
|
2
|
Alcohol, tobacco, smoking
|
1
|
2340 ng/µl
|
|
3
|
1
|
2
|
Smoking ,tobacco
|
1
|
2655 ng/µl
|
|
4
|
1
|
2
|
Smoking, alcohol
|
2
|
135 ng/µl
|
|
5
|
1
|
2
|
Tobacco
|
1
|
3975 ng/µl
|
|
6
|
1
|
4
|
Tobacco
|
2
|
6185 ng/µl
|
|
7
|
1
|
2
|
Alcohol, tobacco
|
2
|
5375 ng/µl
|
|
8
|
1
|
2
|
Smoking, tobacco
|
1
|
430 ng/µl
|
|
9
|
1
|
2
|
Alcohol, tobacco, smoking
|
1
|
1415 ng/µl
|
|
10
|
2
|
2
|
Tobacco
|
1
|
1610 ng/µl
|
|
11
|
2
|
2
|
Tobacco
|
1
|
1780ng/µl
|
Sex: 1=Male (82%), 2=Female (18%) ; Age: <25=1,
26-50=2, 50-75=3, >75=4 ; Purity: 1.8-2.2=1, Others= 2.
On analysis of the agarose gel under the UV transilluminator, the 564 bp band of the amplified β-Actin gene was present in the 10 tumor tissues but no band i.e., amplification was observed in sample 4. Amplified product of XIAP gene was not seen clearly in the gel when observed in UV transilluminator. Out of the 11 samples, 05 samples had clear amplification of both caspase-3 and β -Actin, but with different density as shown in the
figure 2, Table 3. While, 05 samples showed only β -Actin amplification (Figure
3) 01 sample did not show any band or gene amplification even after multiple tries. Density analysis of caspase-3 bands of the 05 samples was done by using my image analysis software (Thermo fisher). Detailed results are given in
Table 3.
Table 3. Lane and Band Analysis
|
Lane
|
Volume (Intensity)
|
Area (Pixels)
|
Density (Intensity/ area)
|
Median (Intensity)
|
Local Bkg. Corr. Volume (Intensity)
|
Local Bkg. Corr. Density (Intensity/Area)
|
Rf
|
% Purity
|
% Purity (Lane)
|
|
Lane 1
|
119758659
|
2126
|
56330
|
45489
|
-13056459.00
|
-6141.33
|
0.251
|
100
|
2.37
|
|
Lane 2
|
44585645
|
2552
|
17470
|
20560
|
-115851599.00
|
-45396.40
|
0.250
|
100
|
0.90
|
|
Lane 3
|
104137171
|
2427
|
42907
|
43690
|
-50652631.00
|
-20870.47
|
0.251
|
100
|
2.07
|
|
Lane 4
|
120741684
|
2506
|
48181
|
38678
|
-38140876.00
|
-15219.82
|
0.250
|
100
|
2.43
|
|
Lane 5
|
100074258
|
2398
|
41732
|
38293
|
-50414125.00
|
-21023.41
|
0.252
|
100
|
1.98
|
Figure 1: Purity of isolated DNA (1.8-2.2=1; others = 2)
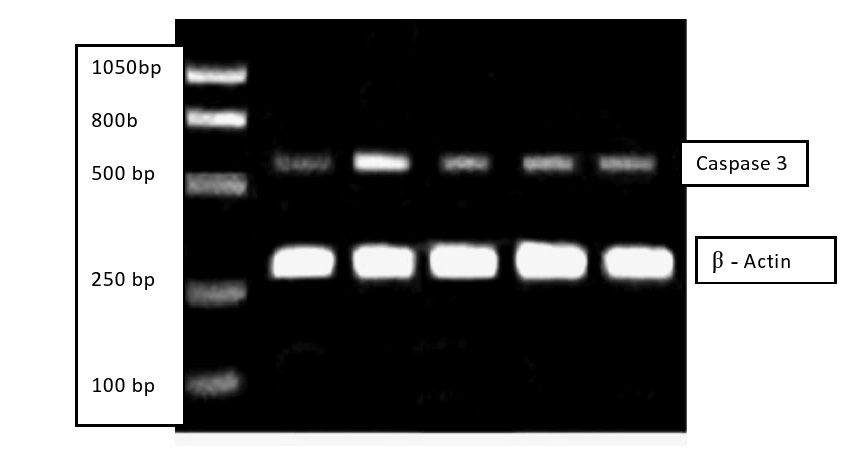
Figure 2: Gel picture showing β-Actin and Caspase
Significantly elevated levels of 8-oxodG excreted in urine were observed in oral cancer patients, with considerable statistical significance. For the whole patient population (n=11), the median values of 8-oxodG in urine samples were 9.47 nM (interquartile range: 5.64–41.13 nmol) which falls between the standard range i.e., 3.13 nmol to 200 nmol (Table
4). In our study, the individual variables such as smoking, alcohol, sex or age, were shown to influence 8- oxodG concentrations. In an attempt to correlate the oxidative stress of oral cancer patients with the history of smoking, tobacco and alcohol, we evaluated the oxidative stress marker 8-oxo-dg in urine. It was observed that the patients, who gave history of smoking and were taking alcohol and tobacco for a long duration, were more prone to DNA damage.
Table 4: Range of the 8-oxodG Standard.
|
Standard No.
|
[8-oxo-dG Standard] (nM)
|
Net Mean A450nm
|
|
1
|
200
|
0.14
|
|
2
|
100
|
0.24
|
|
3
|
50
|
0.4
|
|
4
|
25
|
0.61
|
|
5
|
12.5
|
0.89
|
|
6
|
6.25
|
1.41
|
|
7
|
3.13
|
1.61
|

Figure 3: Gel picture showing β-Actin
Discussion
Apoptosis regulated by caspase cascade (initiator – caspase 8 and 9 and effector caspase 3, 6 and 7) has an intrinsic negative regulation through inhibitors ( IAPs) the only identified proteins interfering with both initiator and effector caspases. Keeping in view of increasing incidence of oral cancers and unpredictable clinical behavior of tumors on basis of histopathology alone, there is an added need for studies aimed to measure biological markers to identify and treat patients with aggressive cancers and poor prognosis for early intervention and increased survival. Our study was focused on studying the less studied expression of XIAP and caspase -3 gene in oral cancer patients which could later lead to establishing preventive and management strategies as there are very few studies which have focused on the role of apoptotic inhibitors in oral cancers. The over expression of XIAP has been reported to be a poorer prognostic factor in various malignancies. However the prognostic value of XIAP in oral cancer patients is yet to be studied with clear conclusions [15,
16]. This study was to the best of our knowledge the first attempt to use PCR through primer designing to determine the expression of XIAP and caspase -3 gene in our part of the world. Out of 11 extracted DNA samples, 07 of our samples showing score 1 were pure DNA samples while four samples having score 2 may have had RNA or protein contamination. Out of 07 pure DNA samples 05 had shown amplification of the caspase-3 gene by PCR though 02 samples did not amplified. The samples in which DNA did not amplify were the DNA with score 2 i.e., they were impure DNA. Further analysis of the five amplified samples revealed clear amplification of caspase-3 gene. The density varied when analyzed by the image analysis software. Impurity in DNA samples may occur due to various reasons such as improper handling of pipette, inadequate mixing of reagents. Low concentration of DNA may also cause impurity as in case of sample 1. Impurity in sample 4 could have been because it was a preserved sample kept in formalin. The few studies done, based on immunohistochemical analysis to evaluate the expression of XIAP in oral cancer conclude that, the XIAP expression was detected in cancer cells and was higher than that in normal cells and the relation could be with histological differentiation or pathological grades [17]. Earlier studies have investigated the impact and expression of survivin a recently discovered IAP and found that in about 80% of oral squamous cell cancers the expression of survivin correlates with aggressive tumor phenotype while the normal oral mucosa did not express survivin [18]. This study is a novel approach to co-relate the XIAP and caspase-3 gene, where we performed molecular analysis of both genes by PCR method. For the confirmation of the gene expression, the gene should be in non-mutated form; in this study we tried to analyze the gene region of XIAP & caspase-3 isolated from oral cancer tissue by polymerase chain reaction before their expression analysis. The study was designed using two markers, apoptotic (caspases) and other anti-apoptotic (XIAP). X-linked inhibitor of apoptosis (XIAP) is a member of the inhibitor of apoptosis protein family that is associated with cell survival by blocking caspase-mediated apoptosis. Molecular Analysis of the pictures captured by the gel documentation of amplified product showed that caspase-3 and the house keeping gene (β-Actin) amplified properly. However, XIAP gene did not amplify in any of the pure DNA samples (score 1) as expected and there was a need to repeat the process which could not be done due to financial constraints. As internal control which is the house keeping β-Actin gene amplified in the same conditions it was presumed that the template DNA/primer/reagent concentration and PCR programme was appropriate. In a recent study from Japan, in samples from patients of oral cancer (biopsy or surgical resections), immune-histochemical evaluation of expression of cleaved caspase-3 was done.The conclusive fact of the study, that the oral cancer cells could be recognized by the expression of caspase-3 and may have clinical and therapeutic implication in oral cancer again emphasizes the need for studying the molecular markers in oral cancer, a similar effort in our study [19]. A study on the expression of XIAP (anti-apoptotic marker) and its correlation with Ki-67 expression (proliferative marker) in benign and malignant salivary gland tumors concluded that one of the promising treatment options for metastatic, drug resistant salivary gland malignancies could be through reversal of XIAP actions [20]. The authors also suggested that continuing studies for other tumors also for evaluation of XIAP expression with larger sample size could throw light on breakthrough in combating the cancers through targeted therapy.
In order to measure the effect of endogenous oxidative damage to DNA, the biomarker 8-oxodG was studied in the urine samples of patients included in our study. The biomarker has been shown as a pivotal marker for not only oxidative stress but also as a risk factor of initiation and promotion of carcinogenesis after exposure to tobacco smoke, asbestos fibers, heavy metals, and polycyclic aromatic hydrocarbons. This biomarker is generated as a result of oxidation of DNA [21]. A study reported a significantly higher level of 8-oxodG in circulating blood cells from esophageal cancer patients compared to control subjects. The study raised a question if oxidative stress was the cause or the result of the disease pathology and if 8-oxodG could be used in evaluation of chemoprevention trials related to cancers of the digestive tract [22]. Similar observations have been made for colorectal carcinoma [23] and lung cancers [24]. A more recent study where 8-OHdG level was determined directly in tumor tissues, in patients with colorectal adenocarcinoma and corresponding normal mucosa, concluded that 8-OHdG reflects the local oxidative stress in colon adenocarcinoma tissue together with ageing processes. Due to multifactorial reasons of oxidative changes in DNA bases, its role as a diagnostic and/or prognostic factor in colon adenocarcinoma was not conclusive though [25]. A double immunofluorescence labeling study in Oral lichen planus (OLP) a chronic inflammatory disease, which has been clinically associated with development to oral cancer has reported accumulation of 8-nitroguanine and 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG) in oral epithelium in OLP and oral squamous cell carcinoma (OSCC) biopsy specimens, whereas little or no immunoreactivity was observed in normal oral mucosa [26]. A case-control study has shown that habitual smokers and tobacco chewers amongst the patients of head and neck cancers including oral cancers had increased salivary DNA adduct (8-OHdG) levels along with other oxidative stress markers. The study suggested a strong contribution towards increased DNA oxidation by free radicals [27]. Also a study in 2008 by Rozalski et al. compared a group of cancer patients and healthy volunteers with regard to the amount of 8-oxodG excreted in urine, demonstrating elevated levels of oxidatively modified base in patients when compared with healthy subjects by 50% [28]. In our study we tried to add a marker of oxidative stress to correlate with gene expression of regulators of apoptosis. However, for application purpose in prevention or disease management more future case control studies on a larger sample size with direct measurement and correlation with oxidative stress and risk factors like smoking, alcohol and tobacco would lead to better conclusions.
Conclusion
This study was a preliminary effort to set a process to study the gene expression of less studied XIAP and caspase -3 through PCR which is also a cost-effective technique, where Primer-3 software was used to design the specific primers of the studied genes .We hereby recommend to conduct the study on a larger sample size. Also advance techniques like real time PCR and western blot may be involved for evaluating the expression of XIAP protein and caspase -3 and its role in future target therapy in our patients. As patients are not diagnosed timely and there is a direct relation to cancer survival and current mode of therapies being offered to patients, there is a need to have studies focused on biomarkers in oral cancers. There is also need to identify patients at risk of aggressive disease and expression of XIAP and caspase - 3 could be studied in relation to clinical stages, histological differentiation and classification of invasion mode of tumor cells in oral cancer patients. We have provided a platform for the molecular analysis of the DNA of XIAP and caspase-3 genes which is reproducible and cost effective; also it may serve as a primary stage for the diagnosis of oral cancer. Genetic predisposition, exposure to risk factors, geographical distribution along with the identification of biological markers may lead the future anticancer therapies and research and development for targeted individual treatments in oral cancer.
Authors' Contribution
MS: carried literature search and prepared the draft manuscript.
RB: participated in study design , performed laboratory work and interpreted the results.
SM: participated in study design , performed laboratory work and interpreted the results.
PJ: did literature search and carried the laboratory experiments.
AR: did literature search and carried the laboratory experiments.
MP: conceived the study, participated in design and edited the final manuscript.
All authors read and approved the final manuscript for submission.
Conflict of Interests
The authors declare that there are no conflicts of interests
Ethical Considerations
This study was approved by the Institutional Ethics Committee of the, Bhopal Memorial Hospital and Research Center, Bhopal, MP, India. (Letter no. IEC/01/dir/14 and IEC/02/dir/14). Consent for participation was taken from the patients as per IEC and institutional norms.
Funding
Funded by the intramural grant from institution.
Acknowledgement
We sincerely thank the administration of BMHRC for support.
References
1.Prasad LK. Burden of oral cancer. An Indian scenario. J Orofac Sci. 2014;6:77
[Free
Full text]
2.Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull WHO. 1984; 62:163–82.
[Pubmed]
[PMC
Full text]
3.Saranath D, Khanna A. Current Status of Cancer Burden: Global and Indian Scenario. Biomed Res J. 2014;1(1):1-5.
[Free
Full text]
4.Kulkarani MR. Head and neck cancer burden in India. Int J Head Neck Surg. 2013; 4: 29-35.
[Free
Full text]
5.Obexer P, Ausserlechner MJ. X-Linked Inhibitor of Apoptosis Protein – A Critical Death Resistance Regulator and Therapeutic Target for Personalized Cancer Therapy. Front Oncol. 2014; 4:197.
[Pubmed]
[PMC
Full text]
6. Mansouri A, Zhang Q, Ridgway LD, Tian L, Claret FX. Cisplatin resistance in an ovarian carcinoma is associated with a defect in programmed cell death control through XIAP regulation. Oncol Res .2003; 13:399–404.
[Pubmed]
[PMC
Full text]
7.Akyurek N, Ren Y, Rassidakis GZ, Schlette EJ, Medeiros LJ. Expression of inhibitor of apoptosis proteins in B-cell non-Hodgkin and Hodgkin lymphomas. Cancer. 2006; 107:1844–1851.
[Pubmed]
[Free
Full text]
8.Ramp U, Krieg T, Caliskan E, Mahotka C, Ebert T, Willers R, et al. XIAP expression is an independent prognostic marker in clear-cell renal carcinomas. Hum Pathol.2004; 35:1022–1028.
[Pubmed]
9.Yan Y, Mahotka C, Heikaus S, Shibata T, Wethkamp N, Liebmann J, et al. Disturbed balance of expression between XIAP and Smac/DIABLO during tumour progression in renal cell carcinomas. Br J Cancer. 2004;91:1349– 1357.
[Pubmed]
[PMC
Full text]
10. Zhang S, Ding F, Luo A, Chen A, Yu Z, Ren S, et al. XIAP is highly expressed in esophageal cancer and its downregulation by RNAi sensitizes esophageal carcinoma cell lines to chemotherapeutics. Cancer BiolTher. 2007;6:973–980.
[Free
Full text]
11.Hofmann HS, Simm A, Hammer A, Silber RE, Bartling B. Expression of inhibitors of apoptosis (IAP) proteins in non-small cell human lung cancer. J Cancer Res ClinOncol . 2002; 128:554–560.
[Pubmed]
12. Yu JQ, Bao W, Lei JC. Emodin regulates apoptotic pathway in human liver cancer cells. PhytotherRes. 2013;27(2):251-257.
[Pubmed]
13.Poomsawat S, Punyasingh J, Vejchapipat P. Overexpression of survivin and caspase 3 in oral carcinogenesis. Appl Immunohistochem Mol Morphol. 2014; 22(1):65-71.
[Pubmed]
14.Kumar A, Pant MC, Singh HS, Khandelwal S. Determinants of oxidative stress and DNA damage (8-OhdG) in squamous cell carcinoma of head and neck. Indian J Cancer 2012; 49:309-15.
[Pubmed]
[Free
Full text]
15.Wilkinson JC, Cepero E, Boise LH, Duckett CS. "Upstream Regulatory Role for XIAP in Receptor-Mediated Apoptosis". Mol. Cell. Biol. 2004; 24(16): 7003–14.
[Pubmed] [PMC
Full text]
16.Javagal V, Rai H. Inhibitors of Apoptosis. Strong Supporters for Oral Cancer Progression. J Adv Med Dent Scie Res 2015; 3(1):121-129.
[ProQuest]
17.Tamatani T, Takamaru N, Uchida D, Nagai H, Fujisawa K, Miyamoto Y. The expression of X-linked inhibitor of apoptosis in human oral squamous cell carcinoma and its relationship with clinical factors. In: Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research. 2012 Mar 31-Apr 4; Chicago, IL. Philadelphia (PA): AACR; Cancer Res. 2012:72(8 Suppl): Abstract nr 4954.
[Abstract]
18.Lo Muzio L, Pannone G, Staibano S, Mignogna MD, Rubini C, Mariggio MA et al. Survivin expression in oral squamous cell carcinoma. Br J Cancer. 2003; 89:2244-2248.
[Pubmed]
[PMC
Full text]
19. Heshiki W, Tomihara K, Yamazaki M, Arai N, Nakamori Kand Noguchi M. Constitutive activation of caspase-3 in non-apoptotic oral squamous cell carcinoma cells. Cancer SciTher. 2015; 7(2) 75-80.
[Free
Full text]
20.Bagulkar BB, Gawande M, Chaudhary M, Gadbail AR, Patil S, Bagulkar S. XIAP and Ki-67: A Correlation Between Antiapoptotic and Proliferative Marker Expression in Benign and Malignant Tumours of Salivary Gland: An Immunohistochemical Study. J ClinDiagn Res. 2015; 9(2):EC01-EC04.
[Pubmed]
[PMC
Pubmed]
21.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2' -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ CarcinogEcotoxicol Rev. 2009; 27(2):120-39.
[Pubmed]
22.Breton J, Sichel F, Pottier D, Prevost V. Measurement of 8-oxo-7,8-dihydro-2'-deoxyguanosine in peripheral blood mononuclear cells: optimisation and application to samples from a case-control study on cancers of the oesophagus and cardia. Free Radic Res. 2005; 39(1):21-30.
[Pubmed]
23.Płachetka A, Adamek B, Strzelczyk JK, Krakowczyk Ł, Migula P, Nowak P, et al. 8-hydroxy-2'-deoxyguanosine in colorectal adenocarcinoma--is it a result of oxidative stress? Med SciMonit. 2013; 19:690-5.
[Pubmed]
[PMC
Full text]
24.Gackowski D, Banaszkiewicz Z, Rozalski R, Jawien A, Olinski R. Persistent oxidative stress in colorectal carcinoma patients. Int J Cancer. 2002; 101:395-397.
[Pubmed]
[Free
Full text]
25.Calişkan-Can E, Firat H, Ardiç S, Simşek B, Torun M, Yardim-Akaydin S. Increased levels of 8-hydroxydeoxyguanosine and its relationship with lipid peroxidation and antioxidant vitamins in lung cancer. ClinChem Lab Med. 2008; 46:107-112.
[Pubmed]
26.Chaiyarit P, Ma N, Hiraku Y, Pinlaor S, Yongvanit P, Jintakanon D, et.al. Nitrative and oxidative DNA damage in oral lichen planus in relation to human oral carcinogenesis. Cancer Sci. 2005; 96(9):553-9.
[Pubmed]
[Free
Full text]
27.A Kumar, MC Pant, HS Singh, S Khandelwal . Determinants of oxidative stress and DNA damage (8-OhdG) in squamous cell carcinoma of head and neck. Indian J Cancer.2012; 49 (3): 309-315.
[Pubmed]
[Free
Full text]
28.Roszkowski K, Gackowski D, Rozalski R, Dziaman T, Siomek A, Guz J, et al. Small field radiotherapy of head and neck cancer patients is responsible for oxidatively damaged DNA/oxidative stress on the level of a whole organism. Int J Cancer. 2008; 123: 1964–67.
[Pubmed]
[Free
Full text]

