Case Report

Generalized lymphadenopathy including supraclavicular lymph node involvement: A rare presentation of urothelial bladder carcinoma. A case report with Fine-Needle Aspiration Cytology and review of the literature.
1 Nicoletta Maounis, M.D., Ph.D., M.I.A.C, 2 Zannis Almpanis, M.D., 3 Maria Chorti, M.D., Ph.D., 4 Constantinos Mantzouranis, M.D., 5 Konstantinos Delamarinis, M.D., 6 Xanthi Tsiafaki, M.D., Ph.D., 7 Maria Demonakou, M.D., Ph.D., 8 Eleni Ellina, M.D.
- 1Director, Department of Clinical Cytology Sismanoglio General Hospital, Athens, Greece
& Adjunct Assistant Professor, Department of Pathology and Laboratory Medicine, Drexel University College of Medicine, Philadelphia, PA, USA,
- 2Registrar A , Department of Pathology, 251 Air Force General Hospital , Greece ,
- 3Director, Department of Pathology, Sismanoglio General Hospital, Athens, Greece.
- 4Resident, Department of Respiratory Medicine, Sismanoglio General Hospital, Athens, Greece, ,
- 5Director, Department of Imaging, Sismanoglio General Hospital, Athens, Greece,
- 6Head, Department of Respiratory Medicine, Sismanoglio General Hospital, Athens, Greece,
- 77 Head, Department of Pathology, Sismanoglio General Hospital, Athens, Greece,
- 88Head, Department of Clinical Cytology, Sismanoglio General Hospital, Athens, Greece ,
- Submitted: February 10, 2017
- Accepted: February 22, 2017
- Published:: February 24, 2017
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Introduction
Urothelial carcinoma of the bladder typically metastasizes to the regional lymph nodes and to visceral sites including bones, lung and liver. Metastasis to supraclavicular lymph nodes is extremely rare.
Case Presentation
We describe a 62-yr-old female with a rare manifestation of disseminated urothelial bladder cancer, with an extensive nested component, who presented with generalized lymphadenopathy. This clinical picture would be most compatible with a malignant lymphoma. The primary diagnostic approach was fine-needle aspiration cytology performed on a palpable mass on the left supraclavicular region.
Conclusion
Fine-needle aspiration cytology, in conjunction with immunocytochemistry, can successfully guide the clinicians to identify the primary site of a neoplasm, especially in unusual cases such as ours.
Key Words
FNAC, Urinary bladder, Urothelial carcinoma, nested, Supraclavicular lymph node, Metastasis
Introduction
cancer in the United States. The vast majority of bladder cancers are urothelial carcinomas (UCs) [1]. The nested variant of UC (NVUC), whether pure or mixed, has an aggressive behavior and represents 0.3% of bladder carcinomas [2]. Metastatic spread of UC tends to occur through the lymphatics or blood vessels, the most common site of metastasis being the regional lymph nodes (LNs) [3]. Pathologic specimens from contemporary radical cystectomy series reveal LN metastasis in up to 26% of resected tumors [4]. Distal LNs are less frequently involved and spread to supradiaphragmatic nodes is uncommon [5]. Moreover, supraclavicular LN metastasis is extremely rare [6, 7].
We report a case 62-yr-old female with a rare presentation of disseminated urothelial bladder cancer initially mimicking a lymphoma because of generalized lymphadenopathy including supraclavicular lymph node involvement. Additionally, this case, describes the use of fine-needle aspiration (FNA) as the first line diagnostic procedure that guided the clinicians to the correct diagnosis.
Case Report
A 62-yr-old woman smoker was admitted to our hospital complaining of a palpable mass on the left supraclavicular region. According to the patient the swelling appeared within the previous month. Physical examination was remarkable for a 3x3.5 cm firm palpable mass in the left supraclavicular area. Additionally, right cervical, bilateral axillary and inguinal lymph nodes were noted. Neck computed tomography (CT) revealed a left supraclavicular lymph node block measuring 3x4 cm (Figure 1a), right cervical and bilateral lower jugular chain nodes. The clinical and radiographic findings supported the diagnosis of lymphoma. FNAC of the palpable mass revealed numerous, large, highly atypical, mostly single cells (Figure 1 c, d). Rare small loosely structured clusters were also observed (Figure 2a). In some cells the cytoplasm was delicate, whereas, in others it was abundant and well defined. The nuclei were large, occasionally eccentric, with irregular nuclear membrane, coarsely granular chromatin and prominent, occasionally multiple, nucleoli (Figure 1 c, d and 2b,).
The morphologic features of our FNA smears helped us rule out the diagnosis of lymphoma or small cell lung carcinoma. Although a metastatic melanoma could not be excluded, a metastatic carcinoma of unknown origin was considered most possible. On immunocytochemistry, tumor cells were positive for CK7 (Figure 2 c, d), CKhigh (Figure 2e), and p63 (Figure 2 f), while negative for TTF-1, Melan A, S-100 protein, Estrogen Receptor (ER), GCDFP-15 and Napsin A. The immunocytochemical findings helped us to rule out the diagnosis of melanoma. The cytomorphological and immunocytochemical features of the tumor cells were compatible with a poorly differentiated carcinoma. While we were not able to determine, with certainty, the primary site based on the markers (TTF-1 (-), Napsin A (-), ER (-), GCDFP-15 (-)), in our cytology report we urged the clinicians to include the urinary tract system in their further investigation as the tumor cells were positive for CK7, p63 and CKhigh; an immunoprofile that is consistent with UC among other carcinomas.
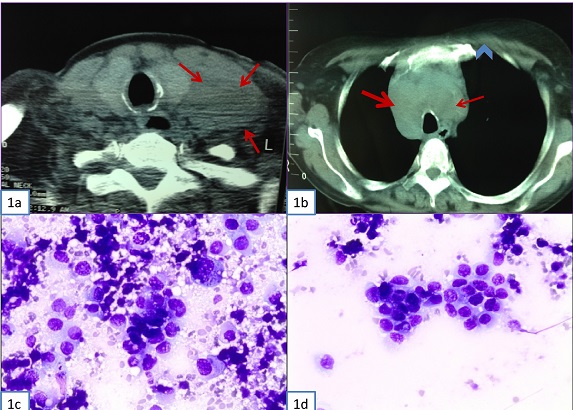
Figure 1: (A) Neck CT. Left supraclavicular lymph node block measuring 3x4 cm. (B) Fig. 1b. Chest CT. Right paratracheal (thick arrow), left paratracheal (thin arrow) and upper mediastinum (arrow head) lymph node enlargement. (C)
& (D) Supraclavicular lymph node fine-needle aspiration cytology. Highly atypical mostly single cells with high nuclear/cytoplasmic ratio, irregular nuclear membrane, coarsely granular chromatin and prominent, occasionally multiple, nucleoli. (Diff Quick stain, x400)
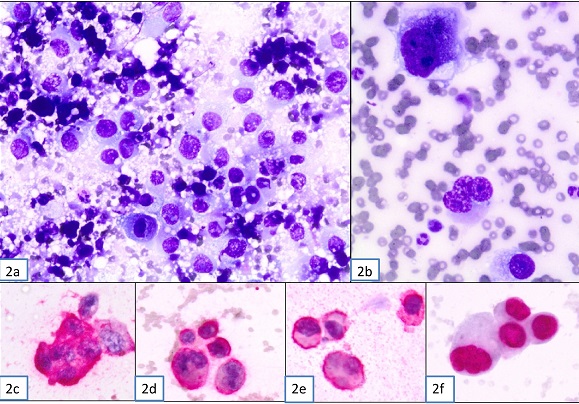
Figure 2: Supraclavicular lymph node fine-needle aspiration cytology. (A) Clusters of malignant cells that exhibit high nuclear enlargement, irregular nuclear membrane, coarsely granular chromatin and prominent, occasionally multiple, nucleoli. (Diff Quick stain, x400), (B) Pleomorphic neoplastic cells and a multinucleated malignant cell. (Diff Quick stain, x600); (C)
& (D) Immunocytochemical CK7 positivity of malignant cells (x400); (E) Immunocytochemical CKhigh positivity of malignant cells (x400) (F) Immunocytochemical nuclear p63 positivity of malignant cells (x400).
CT of the chest and abdomen revealed bilateral upper and lower paratracheal, paraortic, subaortic, hilar, subcarinal, right common and right external iliac LNs (Figure 1b). The lung parenchyma, the liver and the other organs did not demonstrate any evidence of malignancy. The urinary bladder was empty and thus, not examined. Mammography, bronchoscopy, gastroscopy and colonoscopy were performed; the only remarkable finding was enlargement of axillary LNs bilaterally.
Additionally, three voided urine specimens were examined by cytology. The urinary smears revealed numerous, highly atypical cells, mainly single, in an inflammatory background. A few loose clusters were also observed. The cells had irregular configuration and high nucleocytoplasmic ratio. The nuclei were large, hyperchromatic, with irregular contour and coarsely granular chromatin, and occasionally demonstrated enlarged single or multiple nucleoli (Figure 3 a, b). Thus, the diagnosis of a high grade UC was made. Ultrasound showed a large papillary mass at the posterior wall of the bladder measuring 2x2.5 cm. Cystoscopy demonstrated a large protruding mass measuring 2x2.2 cm. Biopsies demonstrated extensive infiltration of the mucosa and muscularis propria of the bladder by a UC mixed type. Thus, the tumor demonstrated histological features both of a conventional high-grade and of a NVUC. (Figure 3 c, d).
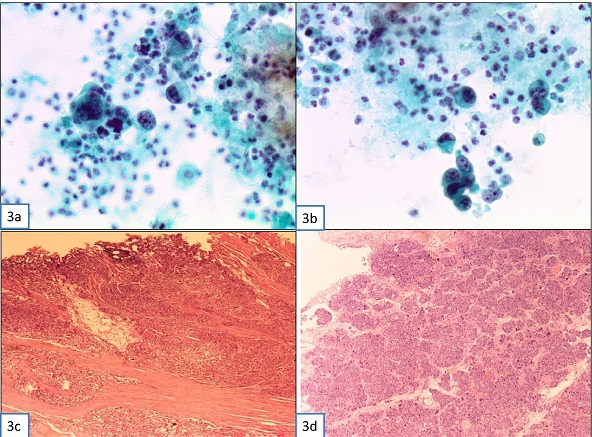
Figure 3: Histology of supraclavicular lymph node excitional biopsy; (A) Metastatic poorly differentiated carcinoma infiltrating the lymph node (Hematoxylin-Eosin, x40); (B) Anaplastic cells with irregular nuclear membrane, coarsely granular chromatin and prominent, occasionally multiple, nucleoli (Hematoxylin-Eosin, x100); (C) Immunohistochemical Uroplakin positivity of malignant cells (x40); (D) Immunohistochemical CK7 positivity of malignant cells (x100).
In order to confirm the extremely rare diagnosis of supraclavicular LNs metastasis by urothelial bladder cancer, as suggested by the FNAC, excisional biopsy of the palpable mass was performed and showed extensive infiltration by an undifferentiated epithelial-like malignant neoplasm (Figure 4 a, b). Immunohistochemistry was positive for uroplakin (Figure 4 c,), CK7 (Figure 4d), focally positive for p63 and CK20, negative for TTF-1, Napsin A, GCDFP-15, Hep Par-1, CK17, mesothelin, CK19-9, CDX-2, Melan A and S-100 protein. Thus, morphological and immunohistochemical analysis confirmed the diagnosis of a metastatic mixed NVUC.
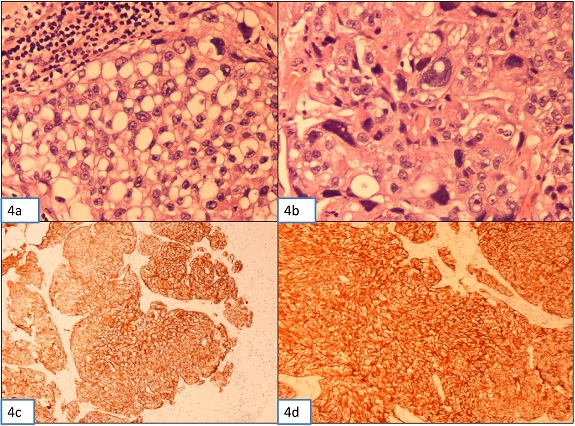
Figure 4: Photomicrograph (A) & (B) Urine cytology, showing highly atypical cells mainly single or in small clusters, in an inflammatory background with irregular configuration and high nuclear/cytoplasmic ratio. The nuclei are large, hyperchromatic, with irregular contour and coarsely granular chromatin and single or multiple prominent nucleoli. (C)
& (D) Histology of the urinary bladder biopsy. Representative Hematoxylin and Eosin-stained images of nested UC associated with traditional UC.
Discussion
Bladder cancer is the most common malignant disease of the urinary tract and represents 4.5% of all new cancer cases in the U.S. UC is the predominant histological type in the United States and Western Europe, where it accounts for approximately 90% of bladder cancers [1].
NVUC is a rare histologic entity with deceptively bland morphologic appearance resembling von Brunn’s nests infiltrating the lamina propria. Studies have suggested that NVUC, seen either in pure form or mixed with a component of traditional UC, has aggressive behavior and poor prognosis [2].
UC of the bladder has variable metastatic potential and almost any organ can be involved [3]. Spread by the lymphatics leads to involvement of the regional LNs in the pelvis and abdomen [3, 5]. Blood – borne metastases occur in the later stages with lesions in the liver, lungs, bones, adrenals, heart, brain and kidneys [3, 7]. In an autopsy study that evaluated 367 cases with muscle invasive bladder cancer, metastases were found in up to 68% of the patients [7]. Additionally, autopsy studies have shown that in 30-40% of patients with bladder cancer, regional LNs may be the only site of metastasis [4].
Metastases to supraclavicular LNs are extremely rare. Our search in the English literature revealed 4 case reports and two series that included 4 patients with cervical LN metastasis of urothelial bladder carcinoma [8-11, 3, 6]. Hessan
et al, reviewed 207 cases of urogenital tract tumors with head and neck metastases and reported only three cases of urothelial bladder cancer that demonstrated metastasis to cervical LNs [6]. In our patient, the presence of enlarged inguinal, intra-abdominal, mediastinal and especially supraclavicular LNs was profound at presentation and thus the first clinical impression was of a lymphoproliferative disease. Additionally, in our case, the diagnosis of extensive LN metastastic disease was not accompanied by organ involvement. A similar clinical presentation was reported only by Kancharla
et al [8].
Cytological examination of voided urine has proved to be an inexpensive, quick, non-invasive and reliable method for identification of the patients who develop high-grade urothelial carcinoma [12]. Although our patient did not report gross hematuria or any other symptoms from the urinary tract, she was diagnosed with a high grade UC by cytology. Thus, urine cytology can play a key role in detecting the urinary system as the primary site of a metastatic carcinoma of unknown origin.
Furthermore, the urine cytology specimens of our patient demonstrated substantial cytologic abnormalities in contrast to the bland morphology of the neoplastic cells described by Cardillo and associates in a series of 7 cases of NVUC [13]. This may be attributed to the traditional UC component observed in our patient’s bladder biopsy.
FNAC has proved to be a safe, accurate and rapid method. FNAC is used widely in the evaluation of lesions in various anatomic sites including the lymph nodes. FNAC, combined with different ancillary techniques, has gained a definitive role in the diagnosis of
benign lymphadenopathies, such as reactive hyperplasia, infections, granulomatous lymphadenopathies, as well as malignant, such as metastatic neoplasms and lymphomas [14-16].
Our case demonstrates that FNAC, in conjunction with immunocytochemistry, can successfully guide the clinicians to identify the primary site of the neoplasm, even in rare and unusual cases, such as ours, where extensive lymphadenopathy was the first sign of the disease.
Authors' Contribution
NM: Cytopathologic reporting, concept, preparation and review of the manuscript.
ZA, MC: Pathologic reporting, design and draft of manuscript.
CM, KD: Literature search and preparation of manuscript.
XT, MD, EE: Review and editing of the manuscript.
All authors read and approved the final manuscript for submission.
Conflict of Interests
The authors declare that there are no conflicts of interests.
Ethical Considerations
Written informed consent for publication of this case report was obtained from the patient.
Funding
None declared
Acknowledgement
None
References
[1]. Bladder Cancer. Patient Version.
National Cancer Institute. US Department of Health and Human Services, National
Institute of Health, USA. http://www.cancer.gov/types/bladder [Last accessed
February 26, 2017]
[2]. Venyo AK. Nested variant of urothelial carcinoma. Adv Urol. 2014;2014:192720. doi: 10.1155/2014/192720. [PubMed]
[PMC Fulltext]
[3]. Shinagare AB, Ramaiya NH, Jagannathan JP, Fennessy FM, Taplin ME, Van den Abbeele AD. Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. AJR Am J Roentgenol. 2011;196:117-122. [PubMed]
[Fulltext]
[4]. Stein JP, Quek ML, Skinner DG. Lymphadenectomy for invasive bladder cancer: I. historical perspective and contemporary rationale. BJU Int. 2006; 97: 227-231.[PubMed]
[Full
text]
[5].Vazina A, Dugi D, Shariat SF, Evans J, Link R, Lerner SP. Stage specific lymph node metastasis mapping in radical cystectomy specimens. J Urol 2004;171:1830-1834.[PubMed]
[6]. Hessan H, Strauss M, Sharkey FE. Urogenital tract carcinoma metastatic to the head and neck. Laryngoscope.1986;96:1352-1356.[PubMed]
[7]. Wallmeroth A, Wagner U, Moch H, Gasser TC, Sauter G, Mihatsch MJ. Patterns of metastasis in muscle-invasive bladder cancer (pT2-4): An autopsy study on 367 patients. Urol Int. 1999;62:69-75.[PubMed]
[8]. Kancharla VP, Gulmi FA, Agheli A, Degen M, Gohari A, Jiang M, et al. Transitional Cell Carcinoma of the Bladder Manifestating as Malignant Lymphoma with Generalized Lymphadenopathy. Case Rep Oncol. 2010;3:125-130.[PubMed]
[PMC Fulltext]
[9]. Pusztaszeri M, Douaihy N, Pelte MF, Pache JC. Cervical lymph node metastasis of a micropapillary carcinoma of the bladder: a case report with fine-needle aspiration cytology and differential diagnosis. Diagn Cytopathol. 2013;41:617-619.[PubMed]
[10]. Bharath C, Komala M. Virchow’s Node: A Look Beyond Gut Carcinoma. Am J Exp Clin Res. 2014;123:33-34.
[DOAJ] [Fulltext]
[11]. Tunio MA, Asiri M, Bayoum Y, Fareed M, Ahmad S. Cervical lymph node metastasis from transitional cell carcinoma of urinary bladder: Case report and review of literature. J of Solid Tumors. 2012:2:59-62.
[Fulltext]
[12]. Bastacky S, Ibrahim S, Wilczynski SP, Murphy WM. The accuracy of urinary cytology in daily practice. Cancer. 1999; 87: 118–128. [PubMed]
[13]. Cardillo M, Reuter VE, Lin O. Cytologic features of the nested variant of urothelial carcinoma: a study of seven cases. Cancer 2003;99:23-27.[PubMed]
[14]. Young NA1, Al-Saleem TI, Ehya H, Smith MR. Utilization of fine-needle aspiration cytology and flow cytometry in the diagnosis and subclassification of primary and recurrent lymphoma. Cancer. 1998;84:252-261.[PubMed]
[15]. Saboorian MH1, Ashfaq R. The use of fine needle aspiration biopsy in the evaluation of lymphadenopathy. Semin Diagn Pathol. 2001;18:110-123.[PubMed]
[16]. Daskalopoulou D1, Harhalakis N, Maouni N, Markidou SG. Fine needle aspiration cytology of non-Hodgkin's lymphomas. A morphologic and immunophenotypic study. Acta Cytol. 1995;39:180-186.[PubMed]

