Original Article

Effect of Bioaccumulation of Gold Nanoparticles on Ovarian Morphology of Female Zebrafish (Danio rerio)
1 Navami Dayal, 2 Dipty Singh, 3Poonam Patil 4 Mansee Thakur 2Geeta Vanage1,3D. S. Joshi
- 1Department of Genetics, MGM Institute of Health Sciences, Navi Mumbai, Maharashtra, India
- 2.National Centre for Preclinical Reproductive and Genetic Toxicology (NIRRH), National Institute ofResearch in Reproductive Health (ICMR), Jehangir Merwanji Street, Parel, Mumbai, Maharashtra, India.
- 3Department of Biotechnology, MGM Institute of Health Sciences, Navi Mumbai, Maharashtra, India.
- 4Department of Biotechnology, MGM CET and Central Research Laboratory, MGM Institute of Health Sciences, Navi Mumbai, Maharashtra, India.
- Submitted: Wednesday, May 25, 2016
- Accepted: TThursday, October 20, 2016
- Published: Thursday, January 5, 2017
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ((http://creativecommons.org/licenses/by/3.0)which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Introduction
Gold nanoparticle's (Au-NPs) have attracted a lot of attention due to their usage in consumer and therapy based biomedical applications. These particles are frequently the medium sized particles mainly within the range of 10-50nm. A number of scientific reports have addressed the cytotoxic potential of these NPs. However, their geotaxis potential with respect to reproductive aspects remains unclear.
Study design
For assessment of safety and risks associated with Au-NPs to female reproductive system, adult female zebrafish (Danio rerio) were exposed in vivo to 20μg/g/day of Au-NPs of two different sizes. AuNPs with average diameter of 15 nm and 47 nm were administered orally to female Zebra fish for a period of 28 days.
Methods
Changes in ovarian morphology is assessed at histopathological level followed by the confirmation of bioaccumulation of Au-NPs using Transmission Electron Microscopy. Using comet assay, strand breaks in DNA of ovarian cells are investigated.
Results
The damage due to accumulated Au-NPs in these cells was found to be statistically significant as compared to those of controls.
Conclusions
In conclusion, Au-NPs of size range 10-50nm are capable of gaining access to ovaries of female zebrafish and potential enough to cause strand breaks in ovarian cells. The present study highlights the adverse effects of these NPs to female reproductive system.
Keywords
Histology, bioaccumulation, gold nanoparticles, zebrafish, ovaries
Introduction
Proof of-concept studies demonstrate various biomedical applications of Au-NPs in chemical sensing [1], biological imaging [2] and drug delivery [3]. Concern lies in the current increase of NP usage mainly in consumer and therapy based applications. Drug delivery using NPs shows tremendous potential but raises concerns regarding local and systemic toxicity [4]. NPs may enter the body via oral ingestion, inhalation, dermal penetration and intravascular injection and subsequently distribute to any organ system. Once NPs enter the body, the misdistribution depends on factors like particle size [5, 6] and surface fictionalization [7]. These particles are subject to first-pass metabolism within the liver where they may accumulate or distribute via the vasculature to end organs including the brain.
The increasing demand of Au-NPs has led to a strong interest in studying their potential to cause deleterious effects in biological systems, and how these effects might be mitigated. This particularly applies to reproductive aspects, where defects can be passed onto the next generations. Repo toxicological studies are a mandatory part during every stage of drug approval processes. are of paramount importance as possible defects may not only affect the person or animal directly treated with the drug but also have possible adverse effects in the following generations. This is not only true for conventional drugs, but also true for NPs. However, very few studies are available that have investigated the effect of NPs on female gametes [8, 9, 10]. The genetic integrity of the gonads is an essential aspect for reproductive success. It is, therefore, necessary to detect DNA alterations in cells of the reproductive tissue.
Despite its obvious importance, repro toxicological testing of NPs has so far been frequently neglected. Most of the cytotoxic and geotaxis effects of NPs have been documented in vitro studies. To clarify these effects, the implementation of in vivo studies has been undertaken using Zebra fish as a model for reproductive toxicity assessments of Au-NPs. In the present study, we investigate the effects of Au-NPs of two different sizes i.e. 15 nm and 47 nm on female reproductive system of Zebra fish after repeated dosing for 28 days (chronic study). The study demonstrates alterations in ovarian morphology at histological level followed by confirmation of bioaccumulation at ultra structural level using Transmission Electron Microscopy. In addition, it also highlights the extent of strand breaks induced by these NPs in ovarian cells based on alkaline comet assay.
Materials and Methods
Synthesis and characterization of Au-NPs
Synthesis and characterization of Au-NPsAu-NPs within the size range 10-20 nm (Type I)were produced following the procedure of Turkevich et al., 1951 [11, 12] while Au-NPs within the size range 40-50 nm (Type II) were produced with few modifications in the procedure of Abdelhalim et al., 2012 [12, 13]. This chemical reduction method for NP synthesis used tetra chloroaurate as metal salt and tir sodium citrate as reducing agent. The size and shape of the NPs were confirmed using Transmission Electron Microscopy (Philip, Model No.CM200, Operating voltages: 20–200 kava resolution 24 A°). The solution was found to be stable for over two months when stored at 4°C.
Adult fish test conditions
All animal experiments were conducted with prior approval from MGM's Medical College, Institutional Animal Ethics Committee. Indigenous wild type Zebra fish strains were maintained at the Zebra-fish facility of MGM Central Research Laboratory. All procedures for maintenance and care of Zebra-fish were as per The Zebra-fish Book [14]. Adult Zebra fish (females weighing 0.4-0.6gm body weight) were used at the age of 4-5 months old. Fish were fed twice a day by local fish feed and live arterial cysts. They were maintained on a 14:10 h light: dark cycle in a room with controlled temperature (28 ± 1ºC).
A total of ninety female fish were randomly divided into nine groups comprising of three control groups and six test groups for each assay. Each group included ten fish. The detailed description of experimental groups is as follows:
Group 1-Histopathology Studies
Group 1A-Control group (n=10)
Group 1B-15 nm Au-NP test group (n=10)
Group 1C-47nm Au-NP test group (n=10)
Group 2-Transmission Electron Microscopy Studies
Group 2A-Control group (n=10)
Group 2B-15 nm Au-NP test group (n=10)
Group 2C-47nm Au-NP test group (n=10)
Group 3-Genotoxicity assessments
Group 3A-Control group (n=10)
Group 3B-15 nm Au-NP test group (n=10)
Group 3C-47nm Au-NP test group (n=10)
Fish from the test groups received oral administration of approximately 100 µl of Au-NP solution at the dose of 20 µg/g/day for 28 days. This dose was determined based on the LC50 value obtained through preliminary screening experiments performed on zebrafish embryos. In contrast, the control groups were administered with equal volume of distilled water. On day 29, fish were sacrificed by anaesthetizing in ice water and dissected to obtain ovaries. The ovaries collected were immediately fixed in respective fixatives for histopathological and electron microscopy studies. In case of genotoxicity assessments, single cell suspensions were prepared for analysis via comet assay.
Histological analysis of ovaries
Histopathological studies
It was of interest to investigate if Au-NPs caused changes in ovarian morphology due to accumulation in ovaries of female Zebra fish. Histological examination was performed following 28 days of chronic exposure. For this purpose, the fish were anaesthetized in ice water and dissected to obtain ovaries. The organs were fixed in 10 % Formalin for 24 hrs at room temperature. Fixed tissue was dehydrated and embedded in the paraffin wax. Serial cross sections of 5 µm were cut by microtome (Leica RM255) and stained with hematoxylin and eosin (H &E). The samples were examined under the light microscope (Olympus Magnus, Model no. 11F589). Staging of germ cells was observed as described by Menke et al., 2003 [15].
Ultrastructural studies
The next step was to confirm the alterations that occurred in ovarian morphology was due to accumulation of Au-NPs. For this purpose, the fish from the control and the test groups were collected for ultrastructural analysis. In order to obtain the ovaries, the fish were anaesthetized and dissected. Ovaries were dissected and fixed in modified Karnovsky’s fixative (4% glutaraldehyde, 4% paraformaldehyde, 0.2% picric acid, 0.02% calcium chloride, 0.2 M cacodylate buffer). Further, the tissues were postfixed in 1% osmium tetroxide in the same buffer, dehydrated in graded acetone solutions, and embedded in resin (araldite). Rinsing, post fixation, dehydration and infiltration was carried out in the KOS microwave tissue processor (Milestone). The ultrathin sections for the TEM analysis (60nm), were obtained using an Leica Ultracut R ultramicrotome (Leica Microsystems, Milton Keynes, England). The ultrathin sections were then stained with 2% alcoholic uranyl acetate and Lead citrate for 10 min in the dark, thoroughly washed in Milli Q water and allowed to air dry before examination. For ultrastructural analysis, the copper grids containing the stained ultra-thin sections were observed under Transmission Electron Microscope (TECNAI, FEI) using an accelerating voltage of 120kV equipped with a CCD camera.
Comet assay
It was evident from histological observations that accumulated Au-NPs in ovaries resulted in degenerative changes in cytoplasm and nuclear material of acolytes and other cell types. This led us to investigate if the accumulated NPs caused DNA damage to ovarian cells. Following 28 days of exposure, cells from ovaries were isolated from each exposure group and the control. In each test group and the control, organs of 3 fish were pooled. For cell isolation, protocols by Braunbeck and Storch, 1992 [16] and Schnurstein and Braunbeck, 2001 [17] for preparation of primary hepatocytes and gill cells in comet assay was applied. Briefly, ovaries from female Zebra fish were dissected and rinsed in 1X PBS, pH 7.2. The tissue was separately incubated in 1X PBS, pH 7.2 and gently teased to release single cells. Filtration step was omitted for female gonads in order to avoid destroying larger oocytes. Cells were then centrifuged for 10 min at 100 g and 20 °C. The cell pellet was then resuspended in chilled 1X PBS, pH 7.2 supplemented with 10 % fetal calf serum at a density of 105 cells/ml.
Cells were checked for viability before the start of the experiment using the Trepan blue dye exclusion test [18]. The comet assay detecting the DNA strand breaks (single and double strand breaks, and alkali labile sites) was performed. The comet assay was performed under alkaline conditions according to Singh et al., 1988 [19] with modifications detailed by Schnurstein and Braunbeck (2001) [20]. Lysis conditions followed the protocol by Kosmehl et al., 2004 [21]. The cells were centrifuged at 1200 rpm/10min and cell pellet was resuspended in chilled 1X PBS, pH 7.2. The suspension (20 µl) was then mixed with 130 µl normal melting agarose (NMA) and placed on a fully frosted microscopic slide precoated witha layer of 1% low melting agarose (LMA). The microscopic slide was then immersed in cold (4 C ) lysis solution. (2.5M NaCl, 100mM EDTA, 10mM Trizma Base. Adjust pH to 10) containing freshly added 1% Triton X-100 and 10% DMSO. After an hour, the slides were dipped in freshly prepared alkaline buffer (1mM Na2EDTA and 300mM Na-Oh, pH 13) for 20min to allow DNA unwinding. Electrophoresis was performed at 50 V for 15-30min. The slides were finally neutralized with a 0.4M Tris Buffer, pH-7.5 and stained with 100 µl Ethidium Bromide (10µg/ml). Observations were made at a magnification of 400X using an inversion fluorescence microscope (Zees) equipped with a 530nm excitation filter, a 590nm emission filter, a digital camera and a comet imager 2.2 analysis software. More than fifty cells from each slide were randomly selected for data analysis of DNA damage percentage in the tail. Experimental data are represented in the form of box and whisker plot. All statistical analysis was performed using Statcalc3 version 4.0. One way ANOVA was used to analyze the significant difference between groups at p<0.05.
Results
Synthesis and characterization of Au-NPs
On characterization using Transmission Electron Microscopy (TEM), the mean diameter of type I &II Au-NPs were 15±6.6nm and 47±7.7nm respectively. The TEM micrographs and size distribution plots for NPs are shown in (Fig1a, b & c d).
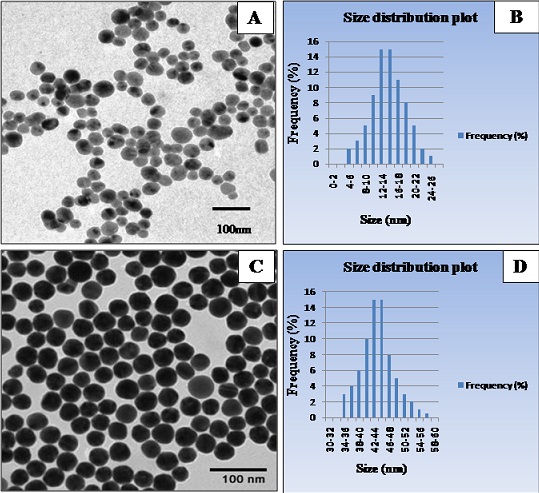
Figure 1: TEM micrographs and size distributin plots for AuNPs formed through chemcial reduction method. (A) and (B) TEM image and size distribution plot for AuNPs indicating the average size diameter to be 15±6.6nm (C) and (D) TEM image and size distribution plot for AuNPs indicating the average size diameter to be 47±7.7nm.
Histopathological examination of ovaries
Six blocks per group were prepared and 10-12 sections per block per observed. Histopathological studies showed normal development of gametogenic populations in the control group. In ovaries of female Zebra fish under control group (Figu2 a, b & c), oocyte/vitellin membrane in the vitellogenic stage appeared regular and intact with easily distinguishable zone radiate and follicular epithelium to the exterior. At every stage of development, there is a proportional increase in the size of the follicles. In the perinucleolar stage (PS), multiple nucleoli were observed at the periphery in the nucleus of the acolytes. The follicle layers were not entirely developed but they were visible. The follicles at this stage of development were comparatively smaller in diameter. In the cortical alveoli’s stage (CASs), the hooplas gets filled with granular structures called cortical alveoli. At this stage, the zona radiate begins to form and the follicular epithelial cells appear to form to the exterior. The next stage of development is the vitellogenic stage (VS), vitellogenesis is seen in the developing follicles. At the final stage of oocyte development i.e. mature stage (MS), marked vitellogenesis is observed in the matured oocyte. Outside the membrane, the follicular epithelial cells were seen with their uniformly arranged nuclei.
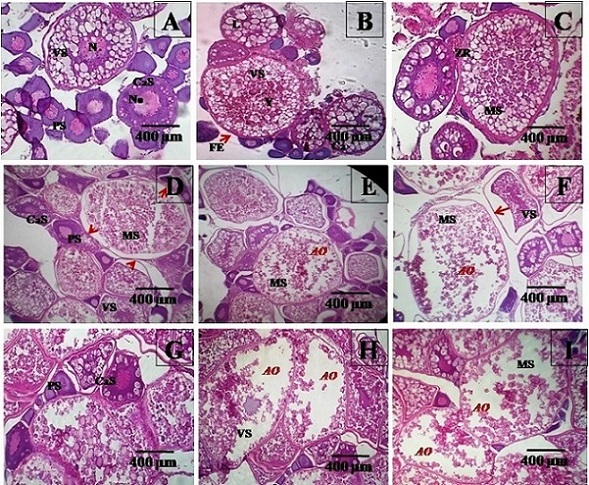
Figure 2: Histological sections of zebrafish ovaries under 100X magnification of optical microscope (A), (B) and (C) Ocytes in different stage sof development in control group. O-Ooplasm, No-Nucleolus, N-Nucleus, PS-Primary stage, CaS-Cortical alveoli stage, Y-Yolk, VS-Vitellogenic stage, ZR-Zona radiata, FE-Follicular epithelial cells and MS-Mature stage. (D), (E) and (F) Ocytes in different stages of development in 15nm AuNP trest group. Primary and Cortical alveoli stage of the oocyte appear normal. Atretic oocytes (AO) can be observed in oocytes at mature stage. Membrane detachments were also seen as marked by red arrow heads. (G), (H) and (I) Ocytes in different stage sof development in 47nm AuNP trest group. Primary and Cortical alveoli stage of the oocyte appear normal. Vitellogenic and Mature oocytes showed gross degenerative changes
For female fish exposed to Au-NPs of 15nm, histology showed detachment of zona radiata from oocyte membrane in oocytes at vitellogenic and mature stage (marked with red arrow heads). However, this condition was not seen in primary and cortical alveoli stage(Fig(2D)). Few atretic oocytes (AO) were also observed particularly in the mature oocyte stage(Fig(2E) & (F)) . Similarly, for female fish exposed to Au-NPs of 47nm, oocytes in primary and cortical alveolar stage appeared normal (Fig(2G)). Atretic oocytes could be seen in both vitellogenic and mature staged acolyte (Fig(2H) & (I)) Irregularity in cell layers could be seen for both the test groups. The number of artistic acolytes quantified for controls were less than 5%. In case of experimental groups, ovaries of 15nm Au-NP treated fish showed 25.01% and those of 47nm treated Au-NP treated fish showed 25.22% artistic acolytes.
Ultra structural examination of ovaries
(Fig(3A) ,(B), & (C)) are representative of electron micrographs of control group. Fig. 3(A) shows an oocyte in its vitellogenic stage (VS) with an intact membrane showing distinct cell layers of theca cells (Th), granulosa cells (Gr) and the zone radiata (ZR), (Fig(3B) ,(C), & (D) shows oocyte in their pprimary oocyte (PS), cortical alveoli (CASs) and mature oocyte stage (MS) respectively. Multiple nucleoli (No) could be observed in primary oocyte stage as shown in Fig. 3(B). Oocyte in Fig. 3(C) indicate cortical alveoli stage with appearance of granular ooplasm. Mature oocytes showed presence of yolk proteins (Y) and lipid droplets (L) as indicated in Fig. 3(D). There were no obvious degenerative changes observed at ultra structural level in all the above mentioned stages of acolytes examined. In contrast, remarkable bioaccumulation of Au-NPs (marked by arrow heads) was seen in the hooplas of both test groups as observed in(Fig(3E) ,& (F)) Electron micrographs of ovary of 15nm Au-NP treated female fish showed abundant Au-NPs aggregates within and surrounding the corticle alveoli as seen in (Fig(3F) at higher magnification than (Fig(3E). Degenerated granulose cells and accumulation of 47 nm Au-NPs in the follicular epithelium of zona radiata can be observed in (Fig(3G)). The marks the ability of 15nm Au-NPs also, to damage the granulose cells of the oocyte membrane and enter the acolytes.(Fig(3H))indicates accumulated NPs around the surface of the lipid vesicles. Apart from bioaccumulation of NPs, degenerative changes were noted at ultra structural level in both the test groups (marked by (*) denotation).
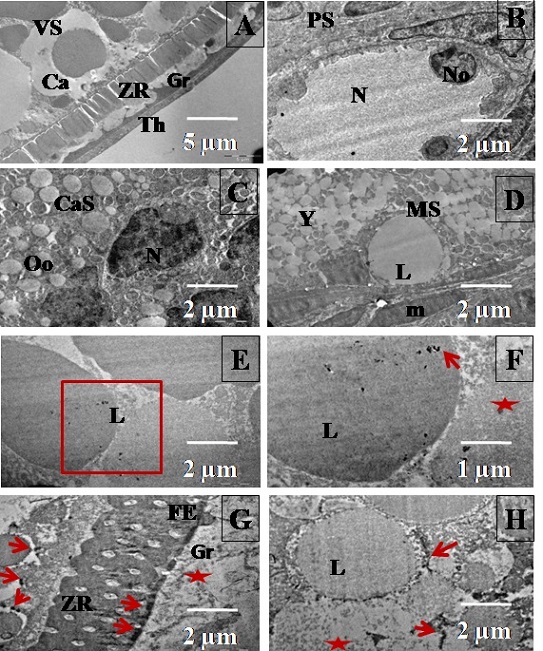
Figure 3: Histological sections of zebrafish ovaries from control (A, B, C and D) and test (E, F, G and H) groups uunder transmission electron microscopy (A) Vitellogenic stage oocyte with an intact membrane showing distinct cell layers of theca cells (Th), granulosa cells (Gr) and the zona radiata (ZR); Scale bar=2µm (B) Oocyte in its primary growth stage (PS) showing multiple nucleoli; Scale bar=2µm (C) Vitellogenic stage oocyte (VS); Scale bar=2µm (D) Mature stage oocyte with yolk (Y) and lipid (L) molecules in the ooplasm; Scale bar=2µm (E) Ovary of zebrafish from 15nm AuNP test group. Oocyte in the vitellogenic stage showing accumulated NPs within and surrounding the lipid vessicles (L); Scale bar=2µm (F) Higher magnification of image (E); Scale bar=1µm (G) and (H) Ovary of zebrafish from 47nm AuNP test group. Degenerated granulosa cells (Gr) are shown in image (G). Mature oocytes showing NPs surrounding the surface of the lipid vesicles (L) and the follicular epithelium of zona radiata are indicated in image (H); Scale bar=2µm.
Genotoxicity assessment in ovarian cells
Accumulation of Au-NPs in female ovaries led to investigate their toxicity to female reproductive system. Genotoxicity assessments were carried out to understand the effects of these accumulated NPs on ovarian cells. The extent of strand breaks in ovarian cells was evaluated using comet assay based on calculating the percentage of tail DNA. For ovaries analyzed from the control group, the proportion of DNA in the tail of ovarian cells from control group was 12 %. The appearance of nucleoids from control group is shown in fig. (Fig(4A) ,(B), & (C)) . Ovarian cells from fish exposed to 15 and 47m Au-NPs respectively showed significant extent of strand breaks. The average extent of strand breaks found in ovarian cells was 51% (Fig(4D),(E) ,& (F)) and 53 % (Fig(4G),(H) ,& (I)) for Au-NPs of average size 15 nm and 47 nm respectively. Statistical analysis using ANOVA showed significant difference in percentage of tail DNA for ovarian cells from both the test groups as compared with that of control group. However, the DNA damage levels did not differ significantly between the two test groups. Percentage of tail DNA is represented in the form of box and whisker plot as described in (Fig 5).
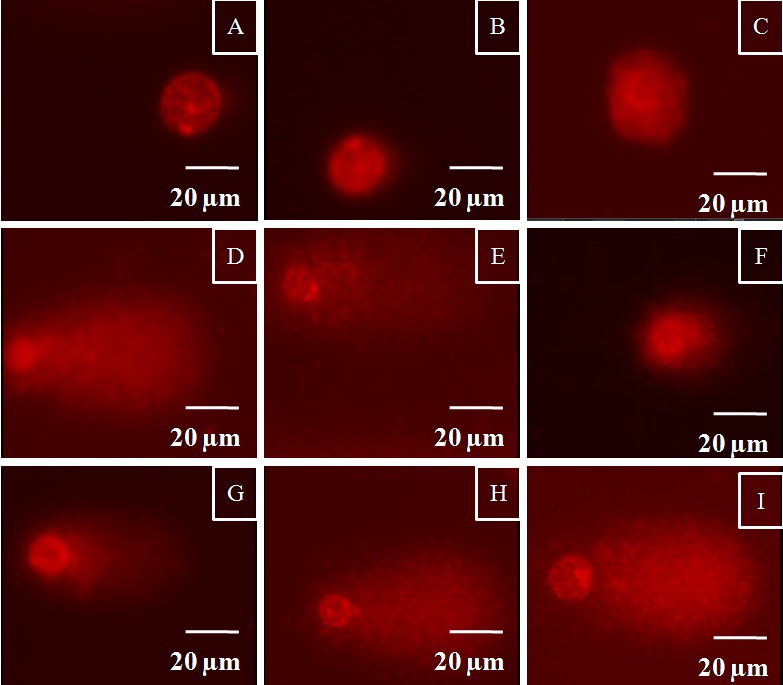
Figure 4: Nucleoids of ovarian cells from zebrafish representing different levels of DNA damage (A), (B) and (C) Control group; (D), (E) and (F) Female zebrafish exposed to 15 nm AuNPs; (G), (H) and (I) Female zebrafish exposed to 47 nm AuNPs.
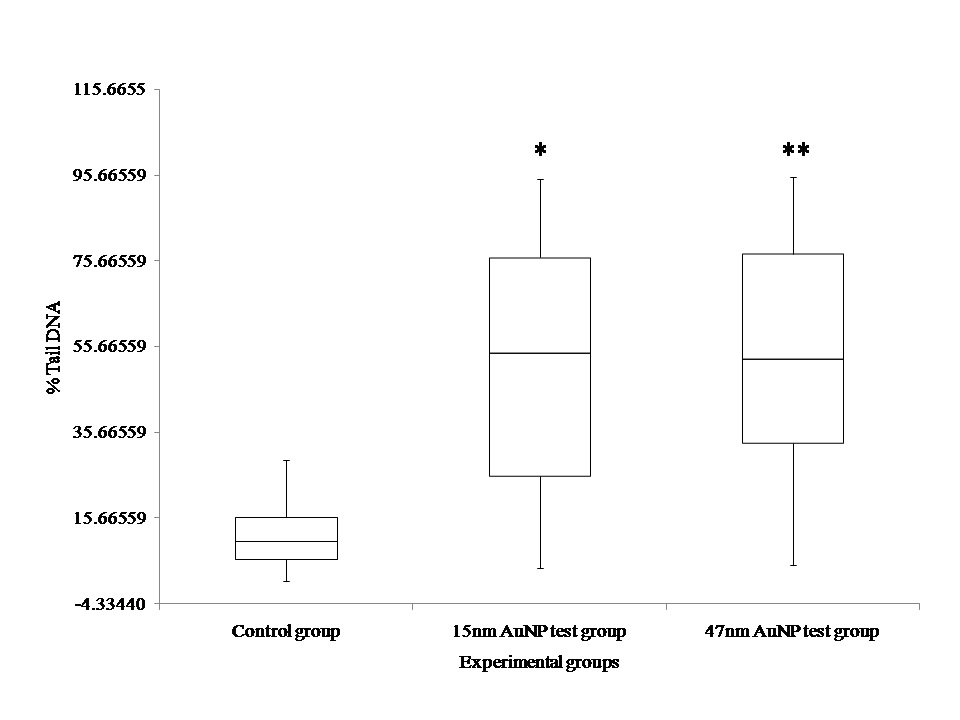
Figure 5: Percentage of comet tail DNA in ovarian cells of D. rerio after 28 days of exposure to 2µg/g AuNPs in vivo. Data are given as means within the box plots. **p<0.01 represent significant differences from control.
Discussion
Exposure to man-made man-sized particles is not a recent event. In the past twenty years technology has evolved sufficiently to allow for the mass production of engineered nanoparticles. Due to their fascinating optical, chemical and physical properties they quickly found their way into many products. With regard to Au-NPs, this applies particularly to biomedical purposes such as cancer imaging, therapy as well as drug delivery [22-24]. This variety in applications generates several potential exposure routes for Au-NPs, including injection and inhalation particularly for biomedical applications, but also ingestion and skin contact for medical and consumer products. The uptake behavior of NPs differs depending on the mode of exposure as well as the particle size. Several reports suggest in vivo toxicity of gold NPs to be directly related to the size, shape, surface coating, exposure dose, and administration routes [25-32]. While many different types of NPs have been tested and found to have geotaxis effects, there remain clear inconsistencies in the reported results. Several recent review articles examining the current literature suggest a number of factors that could contribute to the inconsistent results [33, 34].
The present study aims at studying the female reproductive toxicity associated with Au-NPs of varying sizes. Zebra fish as a model was used in the present study to investigate reproductive toxic effects on female gametes following oral exposure to Au-NPs. The idea of using Zebra fish for reproductive toxicity assessments have recently been adopted by scientists [35-37]. The present study Au-NPs of sizes 15 and 47 nm in an exposure duration of 28 days. Investigations showed accumulated Au-NPs in gonads induced gross alterations in ovarian cells as evident from historical analysis. Few studies have reported the effects of NPs on female gametes. Recently, Tiedemann D, et al., 2014 investigated the response of acolyte complexes to BSA-coated Au-NPs. However, these studies were carried out in vitro. The particles were found to have been internalized in large numbers into the acolytes but did not show any manifestation on acolyte maturation [10]. Hou and colleagues examined the effect of TiO2 NPs on isolated preantral rat follicles in vitro while Hsieh et al.. studied the cytotoxicity of Case quantum dots on the maturation of mouse acolytes, fertilization, and fetal development. Both of these studies reported detrimental effects of NPs on acolyte maturation and further parameters, showing that female gametes are presently at risk when exposed to nana-scale materials [8, 9s].
Bioaccumulation of Au-NPs in reproductive organs may induce DNA damage to the ovarian cells (germ cells as well as other cell types). For this purpose, genotoxicity assessments were carried out using comet assay. The comet assay is a well-established, simple, versatile, visual, rapid, and sensitive tool used extensively to assess DNA damage quantitatively and qualitatively in single cells. The findings in the present study reported presence of strand breaks in ovarian cells (germ cells as well as other cell types). This could be one of the causes for degenerative changes and altered reproductive performance in exposed females. In recent years, studies have evaluated the potential adverse effects of NP exposure on DNA damage to cells of female reproductive system. Zhu et al., 2009 demonstrated toxic effects of TiO2 NPs on CHO cells resulting in DNA strand breaks ]38]. Ha Ryong Kim et al., 2013 revealed Ag-NPs stimulated DNA breakage and micronucleus formation in CHO cells [39]. With regard to overall toxicity, our results agree with the general conception of impairment of the female reproductive function due to metal-based NPs and QDs [40-42]. Wang et al.. 2011 reported chronic exposure to 0.1 mg/L of TiO2-NPs can significantly impair Zebra fish reproduction in females [43]. Interestingly, when Zebra fish (Danio reroof) were exposed to the same NP concentrations for a shorter period of time, female gonads showed a normal spread of acolyte development in all exposed groups [44]. The differences in acolyte development stages observed in these studies could be due to the different exposure periods (13 weeks vs. 14 days) which matches with our findings. A gene expression analysis revealed dramatic transcriptional response indicating that exposure to Ag-NPs has the potential to cause reproductive dysfunction even in the absence of ovary morphological and developmental alterations [45]. These studies demonstrate NPs can interfere with normal female reproductive function by inducing cytotoxic effects on ovarian structural cells, impairing cogenesis and follicle maturation, and altering normal sex hormone levels.
Conclusions
In conclusion, irrespective of size of Au NPs (whetehr 15 nm or 47 nm), it is evident that AuNPs in the range of 10-50nm can gain access to ovaries as demonstrated in Zebra fish. As a result of accumulation, gross degenerative changes were observed in ovarian morphology at histological level. Accumulated Au-NPs were found to induce strand breaks in ovarian cells which include the germ cells of Zebra fish in addition to other cell types. The findings in the present study opens up further avenues for research on effects of these NPs on F1 generation descending from the exposed fish.
Authors 'Contributions
Ms. Navami Dayal is a PhD student who conducted the experimental part comprising of synthesis and characterization of Au-NPs, histopathology and comet assay under the supervision of Dr. Mansee Thakur. Ms. Dayal also contribute to manuscript preparation. Dr. Dipry Singh conducted the electron microscopy analysis of zebrafish ovaries. Ms. Poonam Patil carried out all the dissection process of zebrafish and assisted Ms. Dayal in comet assay procedure. Comet assay procedure was conducted under the guidance and expertise of Dr. Geeta Vanage. Dr. D. S. Joshi contributes to conception of the research work and shares his expertise in nanotechnology.
Acknowledgements
The authors would like to thank MGM Institute of Health Sciences for providing the infrastructure. Special thanks to Mr. Runit Patil, Laboratory Attendant for his efforts in maintenance of the zebrafish facility.
References
[1].Wang C, Yu C. Detection of chemical pollutants in water using gold nanoparticles as sensor. Agricultural and Biosystems Engineering. 2013;32(1):1–14.
[2].Nune SK, Gunda P, Thallapally PK, Lin Y-Y, Laird Forrest M, Berkland CJ. Nanoparticles for biomedical imaging. Expert Opinion on Drug Delivery. 2009;6(11):1175–1194. [Pubmed]
[3].Ajnai G, Chiu A, Kan T, Cheng C-C, Tsai T-H, Chang J. Trends of gold Nanoparticle-based drug delivery system in cancer therapy. Journal of Experimental &Clinical Medicine. 2014;6(6):172–178.
[4].Liu Z, Tabakman S, Welsher K, Dai H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Research. 2009;2(2):85–120 [Pubmed]
[5].De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJAM, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29(12):1912–1919.
[6].Lankveld DPK, Oomen AG, Krystek P, Neigh A, Troost de Jong A, Noorlander CW,et al.. The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials. 2010;31(32):8350–8361.
[7].Lipka J, Semmler-Behnke M, Sperling RA, Wenk A, Takenaka S, Schleh C, et al.. Biodistribution of pEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials. 2010;31(25):6574–6581.
[8].Hou J, Wan X, Wang F, Xu G, Liu Z, Zhang T. Effects of titanium dioxide nanoparticles on development and maturation of rat preantral follicle in vitro. Academic Journal of Second Military Medical University. 2009;29(8):869–873
[9].Hsieh M-S, Shiao N-H, Chan W-H. Cytotoxic effects of CdSe quantum dots on maturation of mouse Oocytes, fertilization, and fetal development. International Journal of Molecular Sciences. 2009;10(5):2122–2135.
[10].Tiedemann D, Taylor U, Rehbock C, Jakobi J, Klein S, Kues WA, et al.. Reprotoxicity of gold, silver, and gold–silver alloy nanoparticles on mammalian gametes. The Analyst. 2014;139(5):931–942.[Pubmed]
[12].Dayal N, Joshi DS and Thakur M. Optimization of citrate method for synthesizing gold nanoparticles of variable particle size and monodispersity Centum 2016;9(1):273-280.
[13].Abdelhalim MAK and El-Toni AM. Optimization of a Citrate Method for Synthesizing GNPs of Controllable Particle Size and Monodispersity: Physical Studies. Open Access Scientific Reports, 2012;1(5): 1-5
[14].Westerfield M. The zebrafish book. A guide for the laboratory use of zebrafish Danio* (Brachydanio) rerio. 4th edition. ZEIF: Univ. of Oregon Press, Eugene; 2000.
[15].Menke AL, Spitsbergen JM, Wolterbeek APM, Woutersen RA. Normal anatomy and histology of the adult Zebrafish. Toxicologic Pathology. 2011;39(5):759–775 [Pubmed]
[16].Braunbeck T, Storch V. Senescence of hepatocytes isolated from rainbow trout (Oncorhynchus mykiss) in primary culture. Protoplasma. 1992;170(3-4):138–159
[17].Schnurstein A, Braunbeck T. Tail moment versus tail Length—Application of an in vitro version of the comet assay in Biomonitoring for Genotoxicity in native surface waters using primary Hepatocytes and Gill cells from Zebrafish (Danio rerio). Ecotoxicology and Environmental Safety. 2001;49(2):187–196.
[18].Strober, W. Trypan Blue Exclusion Test of Cell Viability. Current Protocols in Immunology. 2001;21:3B:A.3B.1–A.3B.2.
[19].Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental Cell Research. 1988 ;175(1):184–191.
[20].Schnurstein A, Braunbeck T. Tail moment versus tail Length—Application of an in vitro version of the comet assay in Biomonitoring for Genotoxicity in native surface waters using primary Hepatocytes and Gill cells from Zebrafish (Danio rerio). Ecotoxicology and Environmental Safety. 2001;49(2):187–196.
[21].Kosmehl T, Krebs F, Manz W, Erdinger L, Braunbeck T, Hollert H. Comparative genotoxicity testing of rhine river sediment extracts using the comet assay with permanent fish cell lines (rtg-2 and rtl-w1) and the ames test*. Journal of Soils and Sediments. 2004;4(2):84–94
[22].Sokolov K, Aaron J, Mack V, Collier T, Coghlan L, Gillenwate A, et. Vital molecular imaging of carcinogenesis with gold bioconjugates. Medical Physics. 2003;30(6):1539–1539.
[23].Gannon CJ, Patra C, Bhattacharya R, Mukherjee P, Curley SA. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. Journal of Nanobiotechnology. 2008;6(1):2
[24].Han G, Ghosh P, Rotello VM. Functionalized gold nanoparticles for drug delivery. Nanomedicine. 2007;2(1):113–123 [Pubmed]
[25].Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005;1:325-7.[Pubmed]
[26].Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjugate Chem 2004;15:897-900 [Pubmed]
[27].Zhang XD, Guo ML, Wu HY, Sun YM, Ding YQ, Zhang LA, et al.. Irradiation stability and cytotoxicity of gold nanoparticles for radiotherapy. Int J Nanomed 2009;4:165-73 [Pubmed]
[28].2Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, et al.. Size-dependent cytotoxicity of gold nanoparticles. Small 2007;3:1941-9.[Pubmed]
[29].Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol 2008;3:145-50.[Pubmed]
[30].Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G, et al.. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small 2009;5:2067-76.[Pubmed]
[31].Tsoli M, Kuhn H, Brandau W, Esche H, Schmid G. Cellular uptake and toxicity of Au55 clusters. Small 2005;1:841-4 [Pubmed]
[32].Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, et al.. Renal clearance of quantum dots. Nat biotechnol 2007;25:1165-70.[Pubmed]
[33].Doak SH, Griffiths SM, Manshian B, Singh N, Williams PM, Brown AP, et al.. Confounding experimental considerations in nanogenotoxicology Mutagenesis. 2009;24(4):285–293 [Pubmed]
[34].Singh N, Manshian B, Jenkins GJS, Griffiths SM, Williams PM, Maffeis TGG, et al.. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials. 2009;30(23-24):3891–3914 [Pubmed]
[35].Ramsden C.S., Henry T.B., Handy R.D. Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish. Aquat. Toxicol.2013;126:404–413 [Pubmed]
[36].Wang J., Zhu X., Zhang X., Zhao Z., Liu H., George R., Wilson-Rawls J., Chang Y., Chen Y. Disruption of zebrafish (Danio rerio) reproduction upon chronic exposure to TiO2 nanoparticles. Chemosphere. 2011;83:461–467.
[37].Bourrachot S, Brion F, Pereira S, Floriani M, Camilleri V, Cavalié I, Palluel O, Adam-Guillermin C. Effects of depleted uranium on the reproductive success and F1 generation survival of zebrafish (Danio rerio). Aquat Toxicol. 2014, 54:1-11 [Pubmed]
[38].Zhu R.R., Wang S.L., Chao J., Shi D.L., Zhang R., Sun X.Y., Yao S.D. Bio-effects of nano-TiO2 on DNA and cellular ultrastructure with different polymorph and size. Mater. Sci. Eng. C. 2009;29:691–696.
[39].Kim HR, Park YJ, Shin DY, Oh SM, Chung KH. Appropriate In Vitro methods for Genotoxicity testing of silver Nanoparticles. Environmental Health and Toxicology. 2013;28:e2013003.
[40].Gao G., Ze Y., Li B., Zhao X., Zhang T., Sheng L., Hu R., Gui S., Sang X., Sun Q., et al.. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles.
[41].Ramsden C.S., Henry T.B., Handy R.D. Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish. Aquat. Toxicol. 2013;126:404–413 [Pubmed]
[42].Stelzer R., Hutz R.J. Gold nanoparticles enter rat ovarian granulosa cells and subcellular organelles, and alter in-vitroestrogen accumulation. J. Reprod. Dev. 2009;55:685–690.
[43].Wang, J.; Zhu, X.; Zhang, X.; Zhao, Z.; Liu, H.; George, R.; Wilson-Rawls, J.; Chang, Y.; Chen, Y. Disruption of zebrafish (Danio rerio) reproduction upon chronic exposure to TiO2 nanoparticles. Chemosphere 2011, 83, 461–467.
[44].Ramsden, C.S.; Henry, T.B.; Handy, R.D. Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish. Aquat. Toxicol. 2013, 126, 404–413. [Pubmed]
[45].Griffith R.J., Brown-Peterson N.J., Savin D.A., Manning C.S., Boube I., Ryan R.A., Brouwer M. Effects of chronic nanoparticulate silver exposure to adult and juvenile sheepshead minnows (Cyprinodon variegatus) Environ. Toxicol. Chem. 2012;31:160–167.

