Original Article

The Relationship of p16, Ki-67, Bcl-2, P53 and CK20 Immune Expressions with Recurrence in Superficial Bladder Tumors
1Özben Yalçin, 2Yeşim Sağlican, 1Süleyman Özdemir, 3Naziye Özkan, 4Naşide Mangir, 5Funda Eren
- 1Department of Pathology, Şişli Etfal Teaching Hospital, Istanbul, Turkey, 2Acibadem University, Faculty of Medicine, Pathology Department, İstanbul, Turkey, 3Marmara University, Vocational School of Health Related Professions, İstanbul, Turkey, Marmara University, 4Department of Urology, 5Department of Pathology, Faculty of Medicine İstanbul, Turkey
- Submitted Sunday, May 17, 2015
- Accepted: Wednesday, July 29, 2015
- Published: Monday, August 03, 2015
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Background
Superficial urothelial carcinoma of the urinary bladder account for 70-80% of all newly diagnosed bladder cancer cases. Of these, approximately 50-75% will experience a recurrence in 12 months and 20 to 25 % will progress to invasive types during the person's lifetime.
Aim
In the present study, we investigated the relationship between the recurrence rate and the expressions of various genes via immunohistochemical analysis in order to assess their applicability as prognostic factors for clinical behavior and differential diagnosis.
Material and Methods
Methods The antibodies that were used in this study; tumor suppressor gene P53, apoptosis activity determinant bcl-2 cell proliferation marker Ki-67, CK20 cytokeratin isotope and tumor suppressor gene P16. Fifty eight cases diagnosed with primary superficial bladder cancer were investigated.
Results
Recurrence was detected in 62.1% of the cases. Progression to a higher grade tumor was detected in 38.9% of the 36 cases that showed recurrence. A marked statistically significant relationship was found between recurrence and diffuse or no staining with CK20, more than 50% heterogeneous expression with P16, and more than 1% expression with bcl-2. Evaluation of the effect of the CK20, bcl-2 and P16 parameters with logistic regression analysis showed that the model was significant (p<0.05) and more than 1% staining with bcl-2 had an explanatory power of 8.057 times on the recurrence rate. A significant relationship was found between the pathological stage and histological grade of the cases and the CK 20 and Ki 67 expressions.
Conclusion
In conclusion, determining CK20, bcl–2, and p16 expressions with immunohistochemical methods can provide guidance in predicting the recurrence of superficial bladder tumors. Abnormal CK20 staining and Ki-67 expression is related to tumor grade and can be used to support the diagnosis in controversial cases.
Keywords
superficial urinary bladder cancer, recurrence, immunohistochemical analysis
Introduction
Superficial bladder cancers make up 70-80% of bladder cancers. Recurrence occurs within about 12 months in 50-75% of the cases and progression related to the tumor grade is seen in 15-25% [1]. Various studies on the factors affecting recurrence and progression have reported various results regarding their significance. Although there is consensus on the prognostic effectiveness of certain factors, new parameters are still needed.
The aim of the study was to reveal the relationship of CK20, bcl-2, p53, Ki-67 and P16 expressions with recurrence rate in superficial bladder tumors and to investigate the significance of their use as prognostic factors that may identify clinical behavior.
Method
Evaluation of immunohistochemical staining and immune reactivity
The streptavidin biotin-peroxidase immunohistochemical staining method was used to show cytokeratin 20, Ki-67, bcl-2, p53, and p16 expression. The external control was used for each.
Evaluation
The histological types of the cases were reevaluated according to the Urothelial Carcinoma Classification (2004) of the World Health Organization. Cytoplasmic positivity was accepted for CK20(Fig 1, 2 and 3) Bcl-2,(Fig 5) and nuclear positivity for p53, Ki-67(Fig 4) and p16(Fig 6). The sections were scanned with the 10x lens and counting was performed using the 40x objective in 3 different areas where the staining was most intense for p53 6, Ki-67, and p16 evaluation
(Fig 7).
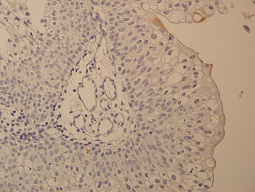
Figure 1: Normal superficial staining with CK20 (400X)
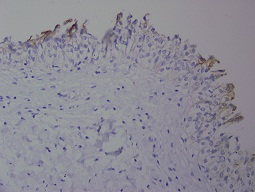
Figure 2: Normal superficial staining with CK20 (400X)
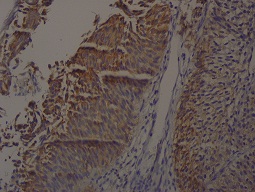
Figure 3: Diffuse staining with CK20 (400X)
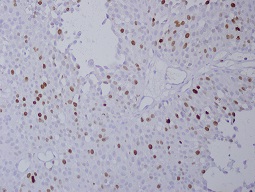
Figure 4: ki-67 immunexpression with more than 50% (400X)
Figure 5: bcl-2 positive cytoplasmic staining (400x)
Figure 6: More than 50% positive with p53 (400X)
Figure 7: >50% strong staining with p16 (400X)
In the normal urothelium, CK20 can show occasional strong staining of umbrella cells. Basal and intermediate urothelial cells were always negative. CK20 immune expression was evaluated with a scale between 1 and 4 as used by Desai [2]. Evaluation scores in our study were as follows:1 = Immune expression in normal superficial cells. 2 = No immune expression; Loss of normal staining 3 = Focal pattern; immune expression in less than 10% of the cells, 4 = Diffuse pattern; immune expression in more than 10% of the cells.
A threshold value of 20-22% has been used in many studies on p53 immune expression [3]. We used :1 = immune expression of 20% or less 2 = immune expression between 20% and 50% 3 = immune expression of more than 50% for the Ki-67 threshold value of 10% was used in many studies [4].
We used:1 = 10% basal immune expression, normal expression. 2 = 10-50% immune expression 3 = Immune expression more than 50%. A threshold value of 1% has been accepted in many studies on Bcl-2 immunohistochemical staining [5]. We used:0 = No staining.1 = Immune expression more than 1%. The study of Shahrokh et al., was used as the basis for p16 [6]: 0 = No nuclear immune expression. 1 = Normal heterogeneous immune expression
2 = Strong homogeneous immune expression of more than 50%
Clinical information
Clinical follow-up information of patients were reviewed; age, sex, tumor location, treatment procedure and recurrence status after the diagnosis were investigated. After transurethral resection (TUR), patients treated with 50 mg Epirubicin within 24 hours, with the clinical diagnosis of superficial bladder tumor. Adjuvant chemotherapeutics were used in principle to prevent the recurrence of low-grade tumors and BCG was used for high-grade tumors according to the European Urology Association Guide, taking into account parameters such as the clinical status, side effects and age.
Clinical data
superficial urothelial carcinoma between 1997 and 2008 in our department were included in the study. The mean age of the patients was 64.41 years (between 32 to 88); 13 (22.4%) of those were females and 45 (77.6%) male. Of these cases, 58.6% had a diagnosis of PUCLG-pTa (Non-invasive papillary urothelial carcinoma, low grade), 17.6% of PUCHG-pT1 (Non-invasive papillary urothelial carcinoma, high grade), 6.9% of PUCLG-pT1 (Invasive papillary urothelial carcinoma, low grade), 5.2% of PUCHG-pT1 (Invasive papillary urothelial carcinoma, high grade ), and 12.1% had other diagnoses Papillary urothelial neoplasm with low malignant potential (5 cases), Inverted Papilloma (1 case) and Papilloma (1 case). The localization of the tumors was; 32.8% in the right wall, 24.1% in the left wall, 15.5% in the posterior part, 15.1% in the bladder neck and 12.1% in the region of the trigone. Recurrence was seen in 62.1% of the cases.
Results
No statistically significant difference was found between the recurrence rates and histopathologic diagnoses, presence of invasion, localization and also depth of invasion (p>0.05). The recurrence rate was higher in low-grade cases than in high-grade ones but the difference was not statistically significant although close to significance (p>0.05)
The evaluation of immune markers and the presence of recurrence
A marked statistically significant relationship was found between recurrence rates and CK20 immune expression levels (p<0.01) (Table 1). The recurrence rate was statistically significantly higher in those with no staining or diffuse staining with CK20. A statistically significant relationship was found between the recurrence rates and Bcl-2 and p16 immune expression level (p<0.01).
Table 1: The evaluation of immune markers and the presence of recurrence
|
|
(+)n (%)
|
(-)n (%)
|
|
|
CK20
|
Normal expresion
|
9 (%36,0)
|
16 (%64,0)
|
0,001**
|
|
No staining
|
11 (%91,7)
|
1 (%8,3)
|
|
Focal patern
|
7 (%58,3)
|
5 (%41,7)
|
|
Diffuse staining
|
9 (%100)
|
0 (%0)
|
|
Kİ-67
|
< 10% staining
|
19 (%52,8)
|
17 (%47,2)
|
0,086
|
|
10-50 % staining
|
12 (%70,6)
|
5 (%29,4)
|
|
> 50% staining
|
5 (%100)
|
0 (%0)
|
|
P53
|
<20% staining
|
4 (%36,4)
|
7 (%63,6)
|
0,125
|
|
20-50% staining
|
6 (%60,0)
|
4 (%40,0)
|
|
>50% staining
|
26 (%70,3)
|
11 (%29,7)
|
|
Bcl-2
|
No staining
|
5 (%29,4)
|
12 (%70,6)
|
0,001**
|
|
More than%1 staining
|
31 (%75,6)
|
10 (%24,4)
|
|
P16
|
Normal expresion
|
10 (%43,5)
|
13 (%56,5)
|
0,018*
|
|
>50% strong staining
|
26 (%74,3)
|
9 (%25,7)
|
|
Chi-Square test is used
|
* p<0.05
|
** p<0.01
|
|
The evaluation of immune markers according to tumor grade
A marked statistically significant difference was present between the CK20 staining levels of the cases according to grade (p<0.01). The rate of 10% staining and diffuse pattern staining with CK20 was significantly higher in high-grade .(Table 2)cases than in low-grade cases. A marked statistically significant difference was present between the Ki-67 staining levels of the cases according to grade (p<0.01). While Ki-67 staining of more than 10% was common in low-grade cases, a Ki-67 staining of more than 50% was significantly more common in high-grade cases than in low-grade cases.
Table 2: The evaluation of immune markers according to tumor grade
|
|
Low graden(%)
|
High graden(%)
|
|
|
CK20
|
Normal Staining
|
21 (%55,3)
|
0 (%0)
|
0,006**
|
|
No staining
|
6 (%15,8)
|
4 (%30,8)
|
|
10 % staining
|
7 (%18,4)
|
5 (%38,5)
|
|
Diffuse staining
|
4 (%10,5)
|
4 (%30,8)
|
|
Kİ-67
|
<10% staining
|
26 (%68,4)
|
3 (%23,1)
|
0,001**
|
|
10-50% staining
|
12 (%31,6)
|
5 (%38,5)
|
|
>50%staining
|
0 (%0)
|
5 (%38,5)
|
|
P53
|
<20% stainimg
|
8 (%21,1)
|
1 (%7,7)
|
0,534
|
|
20-50 % staining
|
6 (%15,8)
|
2 (%15,4)
|
|
>50% staining
|
24 (%63,2)
|
10 (%76,9)
|
|
Bcl-2
|
No staining
|
10 (%26,3)
|
2 (%15,4)
|
0,423
|
|
>1% staining
|
28 (%73,7)
|
11 (%84,6)
|
|
P16
|
Normal staining
|
16 (%42,1)
|
4 (%30,8)
|
0,470
|
|
>50% strong staining
|
22 (%57,9)
|
9 (%69,2)
|
|
Chi-Square test is used
|
** p<0.01
|
|
|
The evaluation of immune markers and presence of invasion
A statistically significant difference was present between CK20 staining levels according to invasion depth (p<0.05). A normal CK20 staining rate was common in cases diagnosed with PTa while a focal and diffuse PT1 staining pattern was significantly more common in PT1 cases. A statistically significant difference was present between the Ki-67 staining levels according to invasion depth (<0.05). A normal Ki-67 staining rate was common in cases diagnosed with PTa while a staining rate of more than 50% was significantly more common with PT1.
The evaluation of immune markers according to the presence of high-grade lesion in recurrence
No statistically significant difference was found between CK20,Ki-67, p53, bcl-2, and p16 immune expression levels of the cases that were and were not high-grade in their recurrence (p>0.05).
Evaluation of immune markers according to the diagnosis and stage
The normal CK20 immune expression rate was high in cases diagnosed with PUCLG-PTa, PUN, Papilloma, and Inverted Papilloma while the focal immune expression rate was high in PUCLG-PT1 and PUCHG-PT, and the diffuse pattern rate in PUCHG-PT1.There was a marked statistically significant difference between Ki-67 immune expression levels among the cases (p<0.01). A statistically significant difference was found between bcl-2 immune expression levels according to histopathological diagnosis (p<0.05).
Statistical evaluation; Logistic regression
Evaluating the effects of the CK20, bcl-2 and P16 parameters with logistic regression analysis showed that the model was significant (p<0.05) and the Negelkerke R square value was 0.225 while the model had a moderate explanatory coefficient (62.2%).(Table 3)The effect of the bcl-2 parameter on the model was found to be statistically significant (p: 0.041; p<0.05 More than 1% staining with bcl-2 had an 8.057 times explanatory power for the recurrence risk.
Table 3: Results of logistic regression analysis
|
|
B
|
S.E.
|
Sig.
|
Exp (B)
|
95.0% C.I.
for EXP(B)
|
|
Lower
|
Upper
|
|
Step
1a
|
CK20(1)
|
,494
|
,794
|
,534
|
1,639
|
,346
|
7,773
|
|
BLC2(1)
|
2,086
|
1,024
|
,041
|
8,057
|
1,084
|
59,892
|
|
P16(1)
|
-,750
|
,953
|
,431
|
,472
|
,073
|
3,058
|
|
Constant
|
-1,383
|
,667
|
,038
|
,251
|
|
|
a. Variable (s) entered on step 1:ck20, BCL2,P16.
Statistical Examinations
The NCSS (Number Cruncher Statistical System) 2007 and PASS 2008 statistical software (Utah, USA) were used for statistical analyses when evaluating study findings. The Chi-Square test and Fisher’s Exacttest were used in addition to descriptive statistical methods (mean, standard deviation, frequency) when evaluating study data. Logistic analysis was applied for multivariate analysis. Significance was evaluated at the level of p<0.05.
DISCUSSION
Bladder cancer is the most common malignancy of the urinary tract. The worldwide age standardized rate (ASR) is10.1 per 100 000 for men and 2.5 per 100 000 for women [7]. Various factors such as tumor diameter, prior recurrence rate, and category are involved in recurrence [8]. We studied the CK20, p53, bcl-2, Ki-67, and p16 markers that can be used to determine recurrence in superficial bladder cancer
A marked statistically significant relationship was also found between abnormal CK20 staining and tumor grade (p<0.01) and the presence of invasion.CK20 immune expression could therefore be useful indetermining presence of invasion and histological grade and can also used as an important marker in the prediction of recurrence. We believe that identifying diffuse staining as well as no staining with CK20 as abnormal staining patterns will be more accurate, as also suggested in other studies.
Bcl-2, an apoptosis inhibitor, is another factor used in studies to identify the prognosis of bladder carcinoma. Ricardo Gonzalez-Campora et al., found high recurrence and mortality rates in Bax-/bcl-2 positive cases and low rates in negative cases [5]. We found a marked statistically significant difference between the recurrence rates according to the bcl-2 immune expression level in our study (p<0.01). The recurrence rate was statistically significantly high in cases with an immune expression rate of more than 1%. Goyal et al., found that bcl-2 positivity was seen in 85% of muscle invasive papillary urothelial carcinoma as compared to only 52.9% of superficial cases. Cytoplasmic bcl-2 immunopositivity was seen in 42.3% of low grade papillary urothelial carcinoma and 85.7% of high grade cases (P = .001) [11]. No statistically significant difference was found when the relationship of bcl-2 expression and histomorphological parameters were compared in our study.
Various proteins are active in the cell cycle control mechanism. Changes in the p16 protein controlled by G1-CDK activity and the Rb protein regulated by G1-CDK contribute to the development of many cancers [12]. Of the four cell regulators, p16 and pRB were found to be more significantly associated with bladder cancer than P53 and P21 in the study conducted by Sharkh et al., [6]. Hitchings et al., stated that P16 and P53 could be used as independent prognostic factors in terms of disease progression in a study they conducted on PTa and PT1 bladder carcinoma cases. They only found P16 changes to be significant in terms of progression when they evaluated P16, pRb and p53 [13]. İn our study the recurrence rate was significantly higher in cases with high immune expression compared to those with moderate immune expression of P16. P16 was thought to be an important factor for recurrence prediction. However, we found no statistically significant difference in P16 staining levels between PTa and PT1 cases (p>0.05).
Evaluating the effects of the CK20, bcl-2 and P16 parameters on recurrence logistic regression analysis showed that more than 1% staining with bcl-2 was found to have an explanatory power of 8.057 times on recurrence risk. These findings indicate that bcl-2 can help determine the possibility of recurrence.
In the normal urothelium the expression of Ki67 is low and limited to basal layers. In many studies authors agree that it varies considerably with histologic grade and clinical stage [14 15]. Nevertheless, Rodríguez-Alonso et al., found that the correlation of relapse with Ki67 was not significant in their studies [16]. Moyano Calvo et al., found that there was an association of Ki67 with recurrence [17]. We found a marked statistically significant difference between Ki-67 staining levels and tumor grade. A statistically significant difference was also found between Ki-67 staining levels and presence of invasion (p<0.05). A normal Ki-67 staining rate was common in PTa patients while a Ki-67 staining rate of more than 50% was significantly more frequent in pT1 patients. When the grade and stage were evaluated together, a marked statistically significant difference was found between Ki-67 immune expression rates (p<0.01). Less than 10% Ki-67 immune expression was seen in cases diagnosed with PUCLG-PTa, PUCLG-PT1, PUNLMP, papilloma and inverted papilloma while a Ki-67 immune expression rate of more than 50% was found inPUCHG-PT1 cases and a Ki-67 immune expression rate of 10% to 50% in PUCHG-PTa cases. These findings indicate that Ki-67 immune expression can be beneficial as a supportive parameter in the evaluation of grade and presence of invasion.
P53 has been associated with prognostic parameters such as recurrence, tumor progression and metastasis-free interval. In some studies the expression of this antibody was associated with tumor progression [13 16]. Stavropoulos et al., found that p53 did not contribute to predicting the recurrence and progression of superficial bladder tumors [18]. No statistically significant difference was found between the recurrence rates according to the immune expression level of p53 in our study. Tony T Wu et al., found no correlation between p53mutation and the stage and grade in their study on p53 expression and grade and invasion depth [19]. We also found no correlation between p53 status and the stage and grade (p>0.05).
Conclusion
It is quite important to determine which superficial bladder tumor cases will recur so that decisions can be made about patient follow-up. The histomorphological characteristics of the tissue alone are not adequate for predicting superficial bladder tumor recurrence. Our study showed that combined use of CK20, bcl–2, p16 could be useful in predicting tumor recurrence. We also believe that Ki-67 and CK20 immune expressions could be helpful in determining tumor grade and stage in superficial bladder carcinomas.
Authors' Contribution
ÖY: carried out the literature search, carried out the experiments and interpreted the results and prepared the draft manuscript.
YS: designed the study and performed the analysis
SÖ: carried out the literature search and prepared the draft manuscript.
NÖ: carried out the experiments and interpreted the results.
NM: conceived the study, participated in design and edited the final manuscript.
FE: conceived the study, participated in design and edited the final manuscript.
Conflict of Interests
The authors declare that there are no conflicts of interests.
Ethical Considerations
The study was approved by the institute ethics committee.
Funding
None declared
Acknowledgement
None
References
[1].Oosterlinck W, Lobel B, Jakse G, Malmström PU, Stöckle M, Stenbeg C: Gui Bladder Cancer Working Group. EAU Guidelines; 2004.[PubMed]
[2].Desai S, Lim SD, Jimenez RE, Chun T, Keane TE, McKenney JK, Zavala-Pompa A, Cohen C, Young RH, Amin MB. Relationship of cytokeratin 20 and CD44 protein expression with WHO/ISUP grade in pTa and pT1 papillary urothelial neoplasia. Mod Pathol 2000; 13(12): 1315-1323.[PubMed]
[3].Salinas-Sanchez AS, Lorenzo-Romero JG, Gimenez-Bachs JM, Sanchez-Sanchez F, Donate-Moreno MJ, Rubio-Del-Campo A, Hernandez-Millian IR, Sequra-Martin M, Atienzar-Tobarra M, Escribano-Martinez J. Implications of P53 gene mutations on patient survival in transitional cell carcinoma of the baldder. Urologic Oncology:Seminars and Original Investigations 2007(July 2007).
[4].Kunju LP, Lee CT, Montie J, Shah RB. Utility of cytokeratin 20 and Ki-67 as markers of urothelial dysplasia. Pathol Int 2005; 55:248–54. [PubMed]
[5].Touloupidis S, Fatles G, Kalaitzis C, Giatromanolaki A, Sivridis E, Simopoulos K, Rombis V. The significance of p53 and Bcl-2 overexpression and other prognostic factors in transitional cell carcinoma of the bladder. Int Urol Nephrol 2006; 38:231-236.{PubMed]
[6].Shariat SF, Tokunaga H, Zhou J, Kim J, Ayala GE, Benedict WF, Lerner SP. p53, p21, pRB, and p16 expression predict clinical outcome in cystectomy with bladder cancer. J Clin Oncol 2004. 22(6): 1014-1024.[PubMed]
[7].Ferlay J, Bray F, Pisani P, Parkin DM. Globcan 2002, cancer incidence, mortality and prevalence worldwide. IARC Cancer Base No. 5, v.2.0. Lyon, France: IARCC Press; 2004
[8].Babjuk M, Oosterlinck W, Sylvester R. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Actas Urológicas-Elsevier; 2012.
[9].Alsheikh A, Mohamedali Z, Jones E, Masterson J, Gilks CB. Comparison of the WHO/ISUP Classification and cytokeratin 20 expression in predicting the behavior of low-grade papillary urothelial tumors. World Health Organization / International Society of Urologic Pathology. Mod Pathol 2001; 14(4):267-272.
[10].Barbisan F, Santinelli A, Mazzucchelli R, Lopez-Beltran A, Cheng L, Scarpelli M, van der Kwast T. Strong immunohistochemical expression of fibroblast growth factor receptor 3, superficial staining pattern of cytokeratin 20, and low proliferative activity define those papillary urothelial neoplasms of low malignant potential that do not recur. Cancer 2008; 112(3):636-644.
[11].Burger M, Denzinger S, Hartmann A, Wieland WF, Stoehr R, Obermann EC. Mcm2 predicts recurrence hazard in stage Ta/T1 bladder cancer more accurately than CK20, Ki67 and histological grade. Br J Cancer. 2007; 96(11):1711-1715.[PubMed]
[12].Goyal S, Singh UR, Sharma S, Kaur N. Correlation of mitotic indices, AgNor count, Ki-67 and Bcl-2 with grade and stage in papillary urothelial bladder cancer.Urol J 2014 ;11(1):1238-1247.[PubMed]
[13].Vernell, R., K. Helin, and H. Muller, Identification of target genes of the p16INK4A-pRB-E2F pathway. J Biol Chem 2003. 278(46): 46124-37.[PubMed]
[14].Hitchings AW, Kumar M, Jordan S, Nargund V, Martin J, Berney DM. Prediction of progression in pTa and pT1 bladder carcinomas with p53, p16 and pRb. Br J Cancer 2004; 91: 552-7.[Pubmed]
[15].Shim JW, Cho KS, Choi YD, Park YW, Lee DW, Han WS,Shin SI, Kim HJ, Cho. Diagnostic algorithm for papillary urothelial tumors in the urinary bladder. Virchows Arch 2008; 452(4):353-362.[Pubmed]
[16].Quintero A, Alvarez-Kindelan J, Luque RJ, Gonzalez-Campora R,Requena MJ, Montironi R, Lopez-Beltran A. Ki-67 MIB1 labelling index and the prognosis of primary TaT1 urothelial cell carcinoma of the bladder. J Clin Pathol 2006; 59(1):83-88.[PubMed]
[17].Rodríguez-Alonso A, Pita-Fernández S, Gonzalez-Carreró J, Nogueira-March JL. Multivariate analysis of survival, recurrence, progression and development of metastasis in T1 and T2a transitional cell bladder carcinoma. Cancer 2002; 94(6):1677-1684.
[18].Moyano Calvo JL, Blanco Palenciano E, Beato Moreno A, GutiérrezGonzález M, Pérez-Lanzac Lorca A, Samaniego Torres A, Montano JA,Fernandez Castineiras J.Cadherina E, catenina beta, antígeno ki-67 y proteína p53 en el pronóstico de la recidiva tumoral en los tumores superficiales de vejiga T1. Actas Urol Esp 2006; 30(9):871-878.
[19].Stavropoulos NE, Filiadis I, Ioachim E, Hastazeris K, Tsimaris I, Kalogeras D, stefanaki S, Agnantis NJ. Prognostic significance of p53, bcl-2 and Ki-67 in high risk superficial bladder cancer. Anticancer Res 2002; 22(6B): 3759-3764. [PubMed]
[20].Wu TT, Chen JH, Lee YH, Huang JK. The role of bcl-2, p53, and Ki-67 index in predicting tumor recurrence for low grade superficial transitional cell bladder carcinoma. J Urol 2000; 163(3): 758-760.[PubMed]

