Research

Prognostic Validity of AgNOR in a Diaspora of Maxillofacial Fibro-Osseous Lesions
1 Bacem AE Ottoman, 2 Iman M. Helmy, 3 Ayman A. Amin, 2Mohamed H. El-Malahy
- 1Maxillofacial surgery, Ministry of Health, Cairo, Egypt
- 2Oral Pathology, Faculty of Dentistry, Ain Shams University, Egypt
- 3 Surgical Oncology, National Cancer institute, Cairo University, Egypt
- Submitted: Sunday, March 22, 2015
- Accepted: Tuesday, June 02, 2015
- Published: Tuesday, June 02, 2015
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Fibro-osseous lesions of the jaws comprise a diverse, interesting, and challenging group of conditions that pose difficulties in classification and treatment. Common to all is the replacement of normal bone by a tissue composed of collagen fibers and fibroblasts that contain varying amounts of mineralized substance, which may be bony or cementum-like in appearance. All were stained with H&E to confirm the diagnosis in addition to the argyrophilic nucleolar organizer region staining stain (AgNOR) to conduct the morphometric study. This study investigates 20 cases of fibrous dysplasia (FD), 35 cases of peripheral ossifying fibroma (POF), 20 cases of central ossifying fibroma (COF), 5 cases of ossifying fibromyxoid tumor, as well as 10 cases of normal mucosa as a control group. One-way ANOVA (ANalysis Of VAriance) with post-hoc Tukey HSD (Honest Significant Differences) tests were calculated to compare between the mean AgNOR score of area fraction of the studied lesion. Pearson’s correlation (r) test and its P-Value were calculated between the mean AgNOR score of area fraction and duration of every lesion.
Key-words
Fibro-osseous lesions, AgNOR stain, fibrous dysplasia, ossifying fibroma, morphometric analysis.
Introduction
Fibro-osseous lesions (FOLs) encompass a group of pathologic processes, characterized by replacement of normal bone by cellular fibrous tissue and newly formed osseous, or so-called cemento-osseous, deposits. FOLs affect, most commonly, the jaws and craniofacial bones. All are characterized by the replacement of bone by cellular fibrous tissue with foci of mineralization that vary in amount and appearance [1]. According to Brannon and Fowler [2], fibro-osseous diseases include fibrous dysplasia (monostotic, craniofacial, polyostotic, and McCune-Albright syndrome), ossifying fibroma (peripheral and central), and Osseous dysplasia (periapical, focal, florid, familial gigantiform cementoma). Langdon et al., [3] suggested that certain FOLs of the jaw may represent different stages in the evolution of a single disease process.
The therapeutic management of FOLs varies depending on the actual disease process. To further complicate matters, a number of other disease processes demonstrate clinical, radiographic, and microscopic features that bear resemblance to those encountered in recognized fibro-osseous conditions. The objective of this paper is to review the most current clinicopathologic, radiographic, and molecular studies of FOLs to help the surgical pathologist recognize and diagnose such diverse group of maxillofacial lesions.
Nucleolar organizer regions (NORs) are loops of DNA which transcribes to ribosomal RNA. The NOR-related protein becomes visible in nucleus by a silver-staining technique under a light microscope, and it has been named argyrophilic protein of NOR (Ag-NOR) [4]. Mammalian nucleoli ultrastructurally undertake a fibrillar center, which is probably the site where the primary ribosomal ribonucleic acid (r-RNA) transcript is generated and contains ribosomal DNA, RNA polymerase I and topoisomerase I: the equivalent of the interphase NOR, which is seen at the light microscopic level [5].
Several methods have been proposed to determine the proliferative rate in tumors. Silver staining of argyrophilic nucleolar organizer regions (AgNOR) is considered to be the best and most cost-effective marker to evaluate the proliferative behavior of a lesion [6].
The rapidity of the cell turnover is evaluated by speed of the cell cycle, so as to consider the growth rate of the lesion, which is easily accessed by AgNOR count per nucleus. The amount of AgNOR represents a cell kinetics parameter and can be used for prognostic purposes. AgNOR counts have been demonstrated to offer a predictive index in various malignancies [7]. In order to achieve a definitive standardization of NOR silver-staining, in 1993 the International Committee on AgNOR Quantitation was established and, two years later, the guidelines for AgNOR quantification were defined. The protocol, proposed by the Committee, included staining of the sections in the dark, at a constant temperature of 37◦ C, and recommended using pre-warmed solutions and glassware. However, staining times vary depending on different preparations. In the same vein, morphometric measurements were preferred to counting methods in AqNOR quantification [8].
Despite the international recommendations, only 15.4% applied image cytometry, while the remaining 84.6% employed the counting method out of 1126 papers published in the literature since 1987 concerning clinical applications of the AgNOR method in cyto-histopathology [9].
Materials &Methods
A - Case selection
A survey on ossifying fibroma cases was performed in archival cases from 2008-2013 in some hospitals and institutes comprising the National Cancer Institute (NCI - Cairo), Al-Qasr Al-eini teaching hospital, dental research centers and some approved educational hospitals. The follow up for recurrence was traced. The net result of a total 80 accepted cases was subcategorized histologically in addition to 10 normal cases as follows:
Group A: Control group;
Group B: Fibrous dysplasia;
Group C: Peripheral ossifying fibroma;
Group D: Central ossifying fibroma (with or without peripheral extension); and
Group E: Ossifying fibromyxoid tumor.
B - Clinical assessment
For every case, the clinical, radiographic, and histologic findings were reassessed. Additionally, the cases were stained histochemically by AgNOR to determine the proliferative activity in the lesions.
Clinically, medical history, age, gender, site, duration, laboratory investigations (if any), radiological findings, were charted. The line of treatment and follow up for recurrence were considered as well
C - Histochemical staining procedures (AgNOR)
Four-micron unstained sections were obtained from blocks of the submitted cases to perform the silver staining. The tissue was deparaffinized in several changes of xylene and descending alcohol concentrations. Rehydration was then performed in several changes of distilled water. The tissue was then incubated in acid alcohol (three parts ethanol: two parts acetic acid) for 5 minutes and then rinsed in distilled water for several times.
The sections were then incubated with silver nitrate solution in a dark humidified chamber for 38 min at room temperature 37 ºC. The silver staining solution consisted of two parts of a 50% solution of silver nitrate and one part of 2% gelatin in 1% formic acid solution. Distilled water was used for preparation of all solutions. The sections were then washed in distilled water, dehydrated in graded alcohol and then xylene and cover slipped. Again, the tissue was then ready for examinations. AgNOR-stained sections were examined under the light microscope (Olympus CHT, Optical Co. Ltd, Japan) using 100x Objectives. The condenser was adjusted to change light intensity and allow test visualization of the AgNORs. Measuring AgNOR score of area fraction was performed under 100 using oil immersion per nucleus and per case (100-200 lesional nuclei). The silver stained nucleoli were visualized as brown black discrete dots of variable size within the nuclei. The nuclei stain light yellow and outline of nuclei as well as cells were usually clearly visible.
D - Digitalization of AgNOR slides
Four fields of the most representative areas are captured at magnification power (100x) from the slides by a digital camera mounted on light microscope, Olympus CHT, Optical. Co. Ltd, Japan, to be digitally processed by Image analysis software (Image J 1.42, NIH, USA) as follows:
1.- Images were refined, trimmed and corrected to exclude all but nuclei.
2.- The corrected images were then converted into 8-gray scale type that is automated to the optimal threshold.
3.- Automated counting of AgNOR-positive nucleoli in the selected field is conducted for measuring the degree of modification—the expressive tool of proliferation within the different variants of ossifying fibromas.
4.- After converting the images into 8-bit images, the threshold was adjusted. Eventually, mean area fraction per nucleus, and per case were measured and charted.
E - Statistical analysis
Data was tabulated to be processed electronically via SPSS 16 software. The data was analyzed and histograms were plotted. The arithmetic mean, mode and standard deviation were calculated. To compare the various groups, ANOVA (ANalysis Of VAriance) with post-hoc Tukey HSD (Honest Significant Differences) tests were calculated to compare between the mean AgNOR score of area fraction, which was recorded in every variant. This was followed by calculating Pearson’s correlation (r) test and its p-value between the mean AgNOR score of area fraction and duration of the studied lesions. The significant result of the P-values meant that we reject the null hypothesis and accept the alternate one. A probability (p) of less than 0.05 (two sided) was considered statistically significant.
Results
A total of 80 archival lesions were submitted in this study. The subcategorization of these cases was 20 cases of fibrous dysplasia (FD), 35 cases of peripheral ossifying fibroma (POF), 20 cases of central ossifying fibroma (COF), 5 cases of ossifying fibromyxoid tumor, as well as 10 cases of normal mucosa as a control group.
I. Clinico-pathological findings
The mean Age of POF was 33.46 (SD=10.8) while FD aged 13.95 (SD=2.6), and COF was 16.85 (SD=7.27). The mean Age of OFMT was 38.6 (SD=12.78).
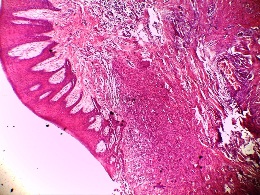
Figure 1: H &E photomicrograph showing the histologic picture of peripheral ossifying fibroma (Original magnification 4
X)
Fibrous dysplasias, on the one hand, have stopped growing in an average time of 3 years while POF showed no significant growth at the same interval. On the other hand, the duration of developing COF and OFMT was way shorter than the other fibro-osseous lesions.
Most cases of COF and FD were young while POF and OFMT preferred the older population. No malignancy could be detected at the time of the study.
Recurrence was evident in 3 cases of COF which was treated by curettage, a single case of OFMT which was treated by wide local excision. No lymph nodes were sonographically assessed and close follow up was recommended.
The histological findings of POF are shown in (Figure 1) where the epithelium is overlying a fibrous stroma with focal ossification. Similarly,(Figure 2,and 3) reveal the typical histological pictures of FD and COF (trabecular variant) respectively.(Figure 5,and 6) depict the histological and histochemical findings of OFMT.
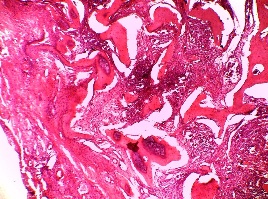
Figure 2: H &E photomicrograph showing the histologic picture of FD.
Original magnification 10 X
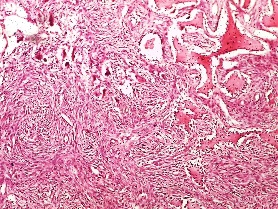
Figure 3: H &E photomicrograph showing the histologic picture of trabecular
central ossifying fibroma with some giant cells. Original magnification 10X
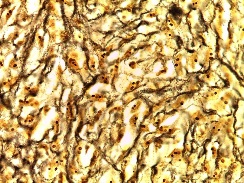
Figure 4: AgNOR stained photomicrograph showing expression of saturated nucleoli. (Original magnification 100X)
II. Morphometric findings (AgNOR scoring)
II. Morphometric findings (AgNOR scoring)AgNOR stained the nucleoli of mononuclear cells and polynuclear cells with dark brown color (Figure 4). The bone was stained with black color. The most representative areas were selected from four locations; the periphery of the lesion, the center of the lesion, and two captures around the formed bone.
The calculated mean AgNOR score of area fraction per nucleus for POF was 0.318 (SD=0.264), comparable to normal mucosa whose mean AgNOR score of area fraction per nucleus was 0.048 (SD= 0.02). However, the mean area fraction of COF per nucleus was 0.581 (SD= 0.43) and the analogous score of OFMT was 1.3612 (SD= 0.22). The pertaining readings of mean AgNOR score of area fraction per case are shown in (Table 1).
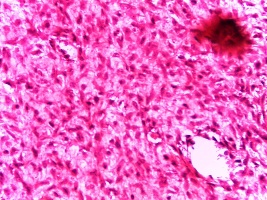
Figure 5: H &E photomicrograph showing the histologic picture of ossifying fibromyxoid tumor. (Original magnification 100X)
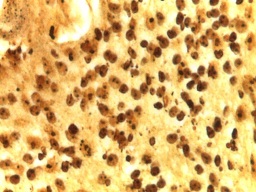
Figure 6: AgNOR stained photomicrograph of the same case showing expression of saturated nucleoli. (Original magnification 100X)
|
Lesion
|
Mean
area fraction/ nucleus
|
Mean
area fraction/ case
|
|
POF
|
0.318 (SD=0.264)
|
0.388286 (SD=0.32)
|
|
FD
|
0.477 (SD= 0.11)
|
0.548 (SD= 0.14)
|
|
COF
|
0.581 (SD= 0.43)
|
0.709 (SD=0.53 )
|
|
OFMT
|
1.3612 (SD= 0.22)
|
1.66 (SD= 0.27)
|
|
Normal
|
0.048
(SD= 0.02)
|
0.63
(SD= 0.003)
|
* p<0.05 : significant and ** p<0.01: highly significant.
III. Statistical Findings
The F-statistic of ANOVA test was 8.1177, whose corresponding p-value was lower than 0.05, suggesting that the one or more treatments are significantly different. The Tukey HSD showed significant differences between all groups except for POF and FD.(Table 2).
|
Study groups
|
Tukey HSD
Q statistic
|
Tukey HSD
p-value
|
|
Normal vs FD
|
4.0585
|
0.0265289 *
|
|
Normal vs POF
|
3.7490
|
0.0468411*
|
|
Normal vs COF
|
6.7877
|
0.0010053**
|
|
Normal vs OFMT
|
15.9139
|
0.0010053**
|
|
FD vs POF
|
0.8272
|
0.8999947
|
|
FD vs COF
|
3.2566
|
0.0100841*
|
|
POF vs COF
|
4.5429
|
0.0100901*
|
|
FD vs OFMT
|
13.1108
|
0.0010053**
|
|
POF vs OFMT
|
14.4217
|
0.0010053**
|
|
FD vs OFMT
|
4.90
|
0.0468411*
|
* p<0.05 : significant and ** p<0.01: highly significant.
To correlate the duration with the measure score of AgNOR, the value of R was -0.6029, a moderate negative correlation, which means there was a tendency for high aggressive behavior (shorter durations) to go with greater score of AgNOR area fraction. The P-Value was < 0.00001. The result was highly significant at p < 0.01.
Discussion
Fibrous dysplasia (FD) is an uncommon but important lesion affecting the maxillofacial region because it can cause severe deformity and asymmetry of face and jaw bones. FD is a genetically-based sporadic disease of the bone; with mutations in the gene (GNAS I) on chromosome 20q13. Thus, FD, as a condition associated with known mutations to the G protein subunit α (Gsα), at 20q13, that couples hormone receptors to intracellular cAMP, results from a localized change in normal bone metabolism that results in the replacement of all the components of cancellous bone by fibrous tissue containing varying amounts of abnormal appearing bone [10].
Taxonomically, FD was mainly divided into two forms, monostotic (single bone involvement) and polyostotic (multiple bone involvement) forms. Polyostotic form is further divided into Jaffe's type and McCune Albright syndrome. In Jaffe-Litchenstein type, variable number of bones plus pigmented lesion of skin 'café au lait' spots are seen. McCune-Albright syndrome is a severe form involving nearly all the bones with pigmented lesions, and endocrine disorder. Another type was identified by Daves and Yardley [11]; craniofacial fibrous dysplasia which involves two or more facial and cranial bones. Eversole et al., reported that the monostotic type occurred in 74% polyostotic type in 13% and craniofacial type in 13% [12].
Radiographically, FD can be investigated by conventional radiography, computed tomography (CT), scintigraphy, and histopathology. Fibrous dysplasia shows various radiographic findings according to the degree of maturation which determines the degree of opacification. Various appearances described on conventional radiographs are radiolucency, ground-glass, smoky, cloudy, peau d'orange, finger print, or diffuse sclerosis [13 14].
The histologic appearance of FD, as with other FOLs, varies depending on the stage of the lesion. Early FD usually exhibits a moderately cellular, fibrous stroma containing haphazardly arranged, uniform, benign-appearing, and spindle-shaped to ovoid fibroblasts. No mitoses are conspicuous. A variable number of small, ovoid blood vessels are seen within the stroma, and collagen bundles often are scant. Scattered, irregularly shaped trabeculae of woven bone are seen throughout the stroma. The trabeculae tend to be delicate and curvilinear and have been likened to Chinese script-writing. Moreover, the lesional bone fuses with the adjacent uninvolved bone—a feature not seen in other FOLs [15]. In our study, fibrous dysplasias have stopped growing in an average time of 3 years. The histological signature of the so-called “Chinese script-writing” was evident in most of the cases. AgNOR expression showed, in this study, comparable numbers to normal mucosa. This should support the inactive nature of fibrous dysplasia. It also poses questions about validity of excision of such lesions.
Ossifying fibroma (OF), by definition, is a well-demarcated lesion composed of fibrocellular tissue and mineralized material of varying appearances. The lesion may grow slowly and might show a very aggressive, rapid and expansile growth. According to WHO classification, histological variants of central ossifying fibroma are; Juvenile trabecular ossifying fibroma and juvenile psammomatoid ossifying fibroma 16
Peripheral ossifying fibroma occurs exclusively on gingiva. It is a relatively common growth of gingiva and is considered to be reactive in nature rather than neoplastic. POF is characterized by a high degree of cellularity usually exhibiting bone formation, although occasionally, cementum-like material or dystrophic calcification may also be found [17]. The POF has a peak incidence in young and teenaged females. Cundiff [18] reported that the lesion is prevalent between ages of 5 and 25 years, with a peak incidence at 13 years of age. He also reported, supporting the classical perspective, a definite female predilection. In our study, POF showed no significant growth at three-year-old interval. On the other hand, the duration of developing either COF or OFMT was way shorter than the other fibro-osseous lesions. AgNOR expression showed, in this study, high numbers in contrast to normal mucosa. This should focu light on the aggressive nature, yet benign, of the central ossifying fibroma. The AgNOR values were inversely proportional to the age of patients at which lesions were encountered.
Central ossifying fibroma is either trabecular ossifying fibroma (TrOF) or psammomatoid ossifying fibroma (PsOF). TrOF consists of cell-rich fibrous tissue containing bands of cellular osteoid without osteoblastic rimming together with slender trabeculae of immature bone containing coarse lacunae with plump osteocytes which are lined by a dense rim of enlarged osteoblasts. Sometimes these trabeculae may anastomose to form a lattice. Mitoses are present, especially in the cell-rich areas. Additional but less typical features are multinucleated giant cells, pseudocystic stromal degeneration, and hemorrhages [19 20].
Psammomatoid ossifying fibroma is significant for multiple round uniform small ossicles (psammomatoid bodies) embedded in a relatively cellular stroma composed of uniform, stellate, and spindle shaped cells. Occasionally, shrunken cells become embedded in the calcified matrix of the ossicles. The psammomatoid bodies are basophilic and bear superficial resemblance to dental cementum, but may have an osteoid rim. Because of a superficial resemblance between these ossicles and the cementum spheres of the odontogenic ossifying fibroma, the lesion has occasionally been mislabeled as cemento-ossifying fibroma, implying an odontogenic origin which, as mentioned above, is rather unlikely in extra- gnathic bone [21]. In our study, All PsOF showed areas of trabecultion, along with psammomatoid bodies, in addition to some myxoid areas.
Ossifying fibromyxoid tumor (OFMT) is a rare soft tissue and bone tumor of unknown histogenesis [22 23]. Ossifying fibromyxoidtumour (OFMT) is a recently described, mesenchymal neoplasm originally defined as a borderline or low-grade malignant lesion. It consists of chains and trabecules of ovoid cells in the fibromyxoid stroma [24]. OFMT is a rare soft tissue tumor of uncertain histogenesis first described in 1989 [25]. Typically, it corroborates a subcutaneous location, lobular architecture and proliferation of small, bland round cells in a fibromyxoid stroma. The most characteristic feature of the tumor, which may permit preoperative recognition, is a peripheral ply or shell of woven bone [26 27]. All in all, most cases of COF and FD were young while POF and OFMT preferred the older population. No malignancy could be detected at the time of the study. Recurrence was evident in 3 cases of COF which were treated by curettage, a single recurrent case of OFMT was traced, which was treated by wide local excision. No lymph nodes were sonographically assessed; yet, close follow up was recommended.
The most intriguing finding of this study is the near histological and histochemical resemblance of both COF and typical OFMT. histochemically, there was no significant differences between TrOF and its sister variant: PsOF.
Conclusions
Peripheral ossifying fibroma and FD, on the one hand, may require no treatment; confirming the reactive rather than the neoplastic nature of these lesions. On the other hand, there are significant relations between POF, COF and OFMT—warranting a rapt attention to the substantially diverse clinical behavior of each. This should prompt new speculations about the validity of concurrent taxonomy of FOLs.
AgNOR staining technique is an excellent diagnostic and prognostic tool in distinguishing subtypes of FOLs. Accordingly, it should be used as an adjunct to the diagnostic procedures.
Conflict of interest
The authors declare that there are no conflicts of interests
Authors’ contributions
IAH and MHE have revised the histological and histochemical findings. IAH has started the drafting of the original manuscript. AAA and BAEO have designed the study and prepared most of the operational framework. BAEO has completed the manuscript writing. IAH and BAEO have run the statistical tests.
All authors have seen the final manuscript and approved it for publication.
Ethical Considerations
The study was approved by the institute ethics committee.
Acknowledgement
None
Funding
References
[1.Speight PM, Carlos R: Maxillofacial fibro-osseous lesions. Current Diag Pathol. 2006; 12:1–10.
[2].Brannon RB, Fowler CB. Benign fibro-osseous lesions: a review of current concepts. Adv Anat Pathol. 2001;8:126-143 [Pubmed]
[3].Langdon JD, Rapidis AD, Patel MF: Ossifying Fibroma-one disease or six? An analysis of 39 fibro-osseous lesions of the jaws. Br J Oral Surg. 1976;14:1–11[Pubmed]
[4].Nakamura S, Takeda Y, Okabe Y, Yoshida T, Ohtake S, Kobayashi K, Kanno M, Matsuda T : Argyrophilic proteins of the nucleolar organizer region in acute leukemias and its relation to the S-phase fraction of leukemic cells. ActaHaematol 1992; 87(1-2):6–10.
[5].Scheer U, Benevente R : Functional and dynamic aspects of the mammalian nucleolus. BioEssays, 1990; 12, 14.[Pubmed]
[6].Bukhari M, Niazi S, Khan S, Hashmi I, Perveen S, Qureshi S, et al : Modified method of AgNOR staining for tissue and interpretation in histopathology, Int J Exp Pathol 2007; 88(1): 47–53 [Pubmed]
[7].Pillai K, Sujathan K, Madhavan J, Abraham E: Significance of silver-stained nucleolar organizer regions in early diagnosis and prognosis of oral squamous cell carcinoma: a multivariate analysis. In Vivo 2005; 19:807–12 [Pubmed]
[8].Aubele M, Bieterferd, S, Derenzini, M, Hufnagl, P, Martin, H, Ofner, D, Ploton, D, Ruschoff, J: Guidelines of AgNOR quantitation. Zentralbl. Pathol. 1994; 140, 107–8 [Pubmed]
[9].Trerè D: AgNOR staining and quantification. Micron. 2000; 31:127–31 [Pubmed]
[10].Waldron C: Bone pathology. In: Neville BW, Damm DD, Allen CM, Bouquot JE, editors. Oral and maxillofacial pathology. Philadelphia: WB Saunders; 1995. 461–65.
[11].Daves M , Yardley J: Fibrous dysplasia of bone. Am J Med Sci. 1957;234:590–606 [Pubmed]
[12].Eversole L, Sabes W and Rovin S: Fibrous dysplasia: a nosologic problem in the diagnosis of fibro-osseous le-sions of the jaws. J Oral Pathol. 1972;1:189–220 [Pubmed]
[13].MacDonald-Jankowski D, Yeung R, Li T and Lee K: Computed tomography of fibrous dysplasia. DentomaxillofacRadiol. 2004;33:114–118 [Pubmed]
[14].Ricalde P, Horswell B: Craniofacial fibrous dysplasia of the fronto-orbital region: a case series and literature re-view. J Oral Maxillofac Surg. 2001;59:157–167 [Pubmed]
[15].Waldron CA. Fibro-osseous lesions of the jaws. J Oral Maxillofac Surg. 1993;51:828-835 [Pubmed]
[16].Leon B, John E, Peter R and David S: WHO pathology and genetics of Head and neck tumors, World Health Organization publication. 2005; 120-143
[17].Rajendran R, Sivapathasundharam B : Shafer’s textbook of oral pathology. 6th ed. Noida, India: Elsevier 2009; 128.
[18].Cundiff E: Peripheral ossifying fibroma A review of 365 cases. USA: MSD Dissertation. Indiana University, 1972; 24-123.
[19].Das N, and Naik S: Trabecular variant of juvenile aggressive ossifying fibroma of anterior mandible. Pediatric Rep 2012; 4(2):e24[Pubmed]
[20].Waldron C, Giansanti J: Benign fibro-osseous lesions of the jaws: a clinical-radiologic-histologic review of sixty-five cases. Oral Surg Oral Med Oral Pathol. 1973;35(2):190-201.
[21].Eversole L, Leider A and Nelson K: Ossifying fibroma: a clinicopathologic study of sixty-four cases. Oral surgery, oral medicine, oral pathology, 1985; 60(5), 505-11[Pubmed]
[22].Makhson A, Bulycheva I, Kuz'min I, Denisov A and Groshev I: Ossifying fibromyxoid tumor . Arkh Pathol 2006; 68(2):41-4 [Pubmed]
[23].Williams S, Ellis G, Meis J and Heffner D: Ossifying fibromyxoid tumour (of soft parts) of the head and neck: a clinicopathological and immunohistochemical study of nine cases. J Laryngol Otol 1993; 107(1):75-80 [Pubmed]
[24].Min K, Seo I and Pitha J: Ossifying fibromyxoid tumor OFMT: modified myoepithelial cell tumor? Report of three cases with immunohistochemical and electron microscopic studies. Ultrastructral Pathology 2005; 29(6):535-48.
[25].Graham R, Dry S, Li X, Binder S, Bahrami A, Raimondi S et al: Ossifying fibromyxoid tumor of soft parts: a clinicopathologic, proteomic, and genomic study. Am J SurgPathol 2011; 35:1615–25. [Pubmed]
[26].Schaffler G, Raith J, Ranner G, Weybora W and Jeserschek R: Radiographic appearance of an ossifying fibromyxoid tumor of soft parts. Skeletal Radiol 1997; 26:615–8.[Pubmed]
[27].Choi J, Park J, Jin W, Park Y and Ryu K: Sonographic features of an ossifying fibromyxoid tumor. J Ultrasound Med 2008; 27:809–12.[Pubmed]

