Case Report

Atypical Carcinoid Tumor of the Lungs: An Enigma
Francis Adedayo Faduyile, Daniel Ayodele Sanni, Sunday Sokunle Soyemi, Olufemi Joshua Taiwo, Adokiye Senebo Benebo
- Department of Pathology & Forensic Medicine, Lagos State University Teaching Hospital, Ikeja, Lagos, Nigeria
- Submitted: April 30, 2012;
- Accepted May 15,2012
- Published: May 24, 2012
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction:
Atypical carcinoid tumor is a rare pulmonary neoplasm, diagnosis of which is commonly missed. Recognition of this disease entity as a possible differential diagnosis of tuberculosis is important in managing patient with this disease.
Case report:
Here we report case of a 53 years old patient who was treated in Lagos, Nigeria as a case of pulmonary tuberculosis and died subsequently of atypical bronchial carcinoid tumor that was never diagnosed antemortem.
Conclusions:
Early detection and high index of suspicion is crucial in the treatment of atypical carcinoid due to poor prognosis, if not treated early.
Introduction
Neuroendocrine tumors of the lung arise from Kulchitzky cells that are normally present in the bronchial mucosa and share the common morphologic features of neuroendocrine tumors including organoid, nesting, palisading, rosettes or a trabecular growth pattern [1]. These tumors represent a broad clinic-pathologic spectrum and have variable morphologic features and biologic behavior [1]. According to the 2004 World Health Organization (WHO) classification of the lung tumors [2], pulmonary carcinoids are classified as typical with low-grade malignancy or atypical with moderate malignancy based on the number of mitotic figures and the presence of necrosis. Typical carcinoids have <2mitoses per mm2 and no tumor necrosis while atypical carcinoids have tumor necroses and 2-10 mitoses per mm2. The large cell neuroendocrine carcinoma which is highly malignant is characterized by more than 10 mitoses per mm2. These tumors account for over 1-2% of all pulmonary neoplasms [1,2]. Such tumors may escape initial detection due to the location and usually small size [3]. Atypical carcinoid tends to have an aggressive clinical course and hence their early diagnosis and treatment is important for a good outcome [3]. We hereby present a case report and autopsy findings of a man who died from atypical carcinoid of the lungs that was being treated as a case of pulmonary tuberculosis.
Case Report
A 53year old clergyman was said to have been ill for more than four months and had several investigations done in many tertiary hospitals in Delta and Lagos States. At a point in time a diagnosis of pulmonary tuberculosis was made and the patient was commenced on adequate doses of antituberculous drugs: Rifampicin, Isoniazid, Pyrazinamide, Ethambutol and Pyridoxine. His condition however continued to deteriorate and he died subsequently at 5:00pm on 29/01/08 at Egbeda, Lagos. The body was brought to the LASUTH morgue for autopsy. Positive history of cigarette smoking, length of illness, time of diagnosis of pulmonary tuberculosis and period on anti-tuberculosis treatment were not available when the patient was brought to LASUTH.
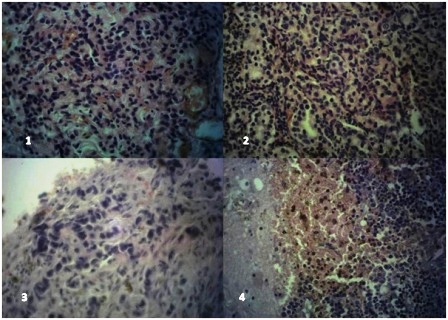
Figure 1: Lung (H & E x400) showing sheets of tumor cells having basophilic and pleomorphic nuclei with many mitotic figures; 2). Chest wall nodule (H&E x400) showing nests, ribbon and festoon of tumor cells with fairly regular nuclei and eosinophilic cytoplasm, up to 5 mitotic figures are seen in this field. 3). Dura mass (H&E x400) showing infiltrating tumor cells in cords with moderately pleomorphic nuclei. 4). Cerebral tissue (H&E x400) showing sheets of tumor cells with basophilic fairly regular nuclei, areas of hemorrhage and haemosiderin pigments are seen
Autopsy Findings
A negroid elderly male who was fairly wasted and mildly pale. The internal organs were in their normal anatomical positions. The left and right lungs weighed 450gm and 500gm respectively and showed moderate congestion with associated edema, there are however diffused grayish white firm masses all over the lobes and they averagely measured 3cm in diameter. The heart weighed 350gm and was normal in size and shape. The heart valvular dimensions were within normal diameter. The kidneys weighed 150gm each with solitary cortical cysts measuring between 0.3 and 0.5cm across and containing clear fluids bilaterally. The brain weighed 1500gm with features of moderate cerebral edema. There was a grayish mass with foci of hemorrhage on the extradural space at the left parietal region. There were no epidural or subdural hemorrhages. The cervical, mediastinal, mesenteric, and para-aortic lymph nodes were all enlarged grayish in color with hard consistency. Cut sections of these lymph nodes revealed whitish grayish surfaces. All other organs appeared unremarkable.
Microscopic Findings
Microscopic features of the grayish white masses were similar in all the sections taken. It showed tumor composed of ovoid, and round cells arranged in sheets, nests, ribbons and festoons. The tumor cells were monomorphic with moderate amount of granular cytoplasm, round nuclei and finely dispersed chromatin with a mitotic rate of about 3/10hpf (Figure 1-4). Focal hemorrhage and foci of haemosiderin pigment deposition were noted. Occasional rosette formation was also seen.
Immunohistochemistry was positive for chromogranin but negative for EMA, CK7, CK20, LCA, S100 and NSE. A diagnosis of atypical carcinoid tumor of the lungs was made with multiple metastases to the lymph nodes and the brain. The primary cause of death was put as atypical bronchial carcinoid tumor with multiple metastases.
Discussion
Carcinoid tumors alone represent 1% to 2% of all lung tumors and 25% of all carcinoid tumors [1,4,5]. Most patients with these tumors have mean age of 46 years [6]. Carcinoid tumors are a type of neuroendocrine tumors which usually involve the upper airways and clinical manifestations of carcinoid include persistent cough, hemoptysis, obstructive pneumonitis, dyspnoea and atelectasis [7,8]. These symptoms however can also be present in a patient with pulmonary tuberculosis [9]. Patients with classic carcinoid syndrome may have intermittent attacks of dyspnoea, flushing and cyanosis [10]. Lymph node metastasis could be a feature of atypical carcinoid tumor [11,12].
Bronchopulmonary carcinoid tumors are relatively uncommon neoplasms and typically benign and slow growing. However, more aggressive subtypes may develop early nodal and distant metastases [13]. Pulmonary neuroendocrine tumors comprise 20% of all lung cancers [14]. They are separated into 4 subgroups: typical carcinoid tumor, atypical carcinoid tumor, large-cell neuroendocrine carcinoma, and small-cell lung carcinoma. They may also exhibit hormonally related symptoms e.g. carcinoid syndrome. Small cell lung cancer is the most common subgroup, with rapid progression, aggressive metastatic potential and the worst prognosis. Large cell neuroendocrine carcinoma is rare but also has a poor prognosis. Typical carcinoid may be accompanied with hormone related symptoms and has the best prognosis; atypical one on the contrary may cause lymph node and distant metastases in half of the cases which was the case in our patient [14].
Carcinoid tumors show histological features of a neuroendocrine tumor characterized by solid nests, ribbon and festoon, rosette-like glands, with the nuclei showing the typical salt and pepper appearance as in (Figure 2). Mitotic figure averaging 2-10/10hpf and presence of necrosis are feature of atypical carcinoid [1,4].
Generally, immunohistochemical methods for carcinoid tumour reveal positive staining for cytokeratin and neuroendocrine markers like synaptophysin, chromogranin and neuron specific enolase [3]. In our case however cytokeratin 7 and 22 were negative and other neuroendocrine markers were also negative except chromogranin. The negative results do not totally rule out the diagnosis because there are different percentages of positivity depending on the tumor sub type. The important differential diagnosis of atypical calcinosis of the lung include small cell carcinoma of the lung which shows neuroendocrine architectural patterns including nested and trabecular growth [15,16], large cell neuroendocrine tumor [17,18], diffuse large cell lymphoma which is a diagnosis consideration in tumors composed predominantly of diffuse sheets, this was however ruled out in this case with the negative result of LCA. The granular character of chromatin, eosinophilic granular cytoplasm, focal nesting pattern and non-reactivity for lymphoid markers also assisted in ruling out this possibility [4].
These tumors are generally resistant to chemotherapy; complete surgical resection is the primary form of therapy. Long-term survival for patients with typical carcinoid is excellent but is decreased in those with the atypical subtype. Complete tumor resection with preservation of uninvolved pulmonary parenchyma remains the fundamental goal in the surgical treatment of this unusual clinical entity [13].
Bagheri et al., in their study showed that the most common surgical procedure is lobectomy or bilobectomy (57.8%) [7]. Bronchial sleeve resection was performed on 10.4% of the patients in their series. They also reported that the most common pathology was the typical form (90%) and 5% of the mediastinal lymph nodes were involved all of the atypical type. They concluded that factors influencing the survival included the pathological type, distant metastasis and mediastinal lymph node involvement [7].
Finally, in our environment disseminated tuberculosis is one of the main differential diagnosis to the symptoms of bronchial carcinoid, physicians must be on alert and strongly investigate for this tumor especially as routine pulmonary surgery are not being practiced in our environment. A biopsy of the lesion for histological evaluation will however confirm the diagnosis.
Conclusions
Atypical carcinoid tumor of the lung is a rare neoplasm with poor prognosis therefore an accurate histopathological diagnosis is essential for its management. It should also be emphasized that it should be considered as a differential diagnosis in cases suspected to be pulmonary tuberculosis, an ailment that is common in our environment.
Authors’ Contributions
FAF: Conceived the study, reviewed the autopsy, carried out literature search, reported the histology slide, drafted and edited the manuscript
DAS: Performed the autopsy, carried out the literature search and edited the manuscript
SSS: Reported the slides, reviewed the autopsy and edited the manuscript
OJT: Carried out the literature search and edited the Manuscript
ASB: Reviewed the histology slide, contributed in editing the Manuscript
All authors have read and endorsed the manuscript.
Conflict of Interest
The authors declare that there are no conflicts of interests.
Ethical Considerations
The consent was obtained from the next of kin of the patient.
References
[1]. Chong S, Lee KS, Chung MJ, Han J, Kwon OJ, Kim TS. Neuroendocrine tumors of the lungs: Clinical, Pathological and Imaging findings. Radiograhics. 2006; 26: 41-57
[2]. Travis WD, Rush W, Flieder DB, Falk R, Fleming MV, Gal AA, Koss MN. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998; 22: 934–44.
[3]. Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC (eds). Tumors of the lung, pleura, thymus and heart. Pathology & genetics. World Health Organization Classification of Tumors. IARC Press, Lyon, 2004
[4]. Moran CA, Suster S, Coppola D, Wick MR. Neuroendocrine carcinomas of the lung: A critical analysis. Am J Clin. Pathol. 2009; 131: 206-221
[5]. Modlin IM, Lye KD, Kidd M. A 5- decade analysis of 13,715 carcinoid tumors. Cancer 2003; 97: 934-959
[6]. Schreurs AJ, Westermann CJ, van den Bosch JM, Vanderschueren RG, Brutel de la Rivière A, Knaepen PJ. A twenty-five-year follow-up of ninety-three resected typical carcinoid tumors of the lung. J thoracic Cardiovasc Surg. 1992; 104: 1470-1475.
[7]. Bagheri R, Mashhadi MR, Haghi SZ, Sadrizadh A, Rezaeetalab F. Tracheobronchopulmonary carcinoid tumors: analysis of 40 patients. Ann Thorac Cardiovasc Surg. 2011; 17: 7-12.
[8]. Fink G, Krelbaum T, Yellin A, Bendayan D, Saute M, Glazer M, Kramer MR. Pulmonary carcinoids: Presentation, Diagnosis and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest. 2001; 119: 1647-51
[9]. Lawson L, Lawson JO, Olajide I, Emenyonu N, Bello CS, Olatunji OO, Davies PD, Thacher TD. Sex differences in the clinical presentation of Urban Nigerian patients with pulmonary tuberculosis. West Afr J Med. 2008; 27: 82-86
[10]. Kvols LK, Moertel CG, O'Connell MJ, Schutt AJ, Rubin J, Hahn RG. Treatment of the malignant carcinoid syndrome; evaluation of a long acting somatostatin analogue. N Engl J Med. 1986; 315: 663-666
[11]. Warren WH, Welker M, Gattuso P. Well differentiated neuroendocrine carcinomas; the spectrum of histologic subtypes and various clinical behavior. Semin Thorac Carciovasc Surg. 2006; 18: 199-205
[12]. Akiba T, Naruke T, Kondo H, Goya T, Tsuchiya R, Suemasu K, Noguchi M, Sakurai K. Carcinoid tumor of the lung: Clinicopathological study of 32 cases. Japan J. Clin Onco. 1992; 22: 92-96
[13]. Wei S, Li X, Chen J, Zhou Q. Diagnosis and therapy of bronchopulmonary carcinoid tumors. Zhongguo Fei Ai Za Zhi. 2011; 14: 733-8
[14]. Tamási L, Müller V. Symptoms and diagnostics of lung neuroendocrine tumors. [Article in Hungarian]. Orv Hetil. 2011; 152: 366-70
[15]. Warren WH, Gould VE. Differential diagnosis of small cell neuroendocrine carcinoma of the lung. Chest Surg Clin N Am. 1997; 7: 49-63
[16]. Takei H, Asamura H, Maeshima A, Suzuki K, Kondo H, Niki T, Yamada T, Tsuchiya R, Matsuno Y. Neuroendocrine carcinoma of the lung: a clinicopathologic study of eighty-seven cases. J thorac Cardiovasc Surg. 2002; 124: 285-292
[17]. Hiroshima K, Iyoda A, Shida T, Shibuya K, Iizasa T, Kishi H, Tanizawa T, Fujisawa T, Nakatani Y. Distinction of pulmonary large cell neuroendocrine carcinoma from small cell lung carcinoma: a morphological, immunohistochemical and molecular analysis. Mod Pathol. 2006; 19: 1358-1368
[18]. Battafarano RJ, Fernandez FG, Ritter J, Meyers BF, Guthrie TJ, Cooper JD, Patterson GA. Large cell neuroendocrine carcinoma: An aggressive form of non small cell lung cancer. J thorac Cardiovasc Surg. 2005; 130: 166-172

