Original Article

A single group study into the effect of intralesional tetra-O-methyl nordihydroguaiaretic acid (M4N) in oral squamous cell carcinoma
1 Madhvan K Nair, 2 ,Manoj Pandey,3 Ru Chih C. Huang
- 1Department of Radiation Oncology, Regional Cancer Centre Thiruvananthapuram, Kerala, India.
- 2Department of Surgical Oncology, Regional Cancer Centre Thiruvananthapuram, Kerala, India.
- 33Department of Biology, Johns Hopkins University Baltimore, MD 21218 USA
- **Presently working in Department of Radiation Oncology, Amritha Institute of Medical Sciences, and Kochi, India.
- Submitted:
- Accepted:
- Published:
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Background:
Oral squamous cell carcinoma is the most common cancer among males and third commonest among females in India. Conventional treatment is by surgery or radiotherapy either alone or in combination. Survival still remains poor with nearly 50% recurring within 2-years of treatment.
Patients and Methods:
Between November 1999 and April 2000, 21 patients with oral squamous cell carcinoma were recruited. Of these, 3 patients did not complete the intended treatment and were excluded from analysis. After obtaining an informed consent, intralesional tetra-O-methyl nordihydroguaiaretic acid (M4N, currently under development as EM-1421) was injected in doses of 20 mg/day for 3 or 5 days. After completion of this treatment, patients underwent surgical resection of the tumor followed by adjuvant radiotherapy if indicated. Toxicity was monitored by WHO toxicity criteria. Response was recorded as change in surface area, tumor necrosis, pathological response, molecular response complication and recurrences. Overall, disease free and event free survival were calculated using the Kaplan-Meier method and were compared using log-rank testing. Survivin and Cdc2 expression in a preoperative biopsy and postoperative surgical specimen was documented using immunohistochemistry. The survivin and Cdc2 data was analyzed using a Chi square test and the effect of change of survivin and Cdc2 expression on survival was evaluated by the Kaplan-Meier method and log-rank test
Results
Of the 18 patients, 7 (38.9%) had pT2 disease, 4 (22.2%) had pT3 disease while 6 (33.3%) had pT4 disease. 9 (50%) of the patients were node negative while 3 (16.7%) had pN1 disease and 4 (22.2%) had N2b disease. Over a period of 5 years, 4 patients died. Two died (11.1%) of non-tumor related causes. Six (33.3%) patients recurred at primary site, neck node or developed second primary tumors. Two of these (11.1%) died due to second primary tumors at sites distinctly separate from the treated tumor. The other four (22.2%) who relapsed were either salvaged or were on palliative treatment. There were no acute or deferred toxicities in any of the patients. Disease free and overall survival at 5 years was 10/18 (55.6) % and 14/18 (71.7%) respectively. Down-regulation of Cdc2 was observed in 9/18 (50%) and of survivin in 10/18 (55.6%) patients. Tumor stage at presentation (p=0.01) and down regulation of survivin (p=0.01) were found to significantly correlate with disease free survival.
Conclusions
Intralesional tetra-O-methyl nordihydroguaiaretic acid (M4N) appears to be well tolerated using an intralesional dose of up to 100mg (20mg / day) with no evidence of acute or deferred toxicity. M4N has demonstrated significant inhibition of cellular production of Cdc2 and survivin, a potent pro-apoptotic response. This effect translated into tumor necrosis and shrinkage even with this short exposure in association with concomitant surgery and / or radiation.
Key words:
Oral cancer; squamous cell carcinoma; M4N, EM-1421, intralesional administration, cdc2, survivin, apoptosis, caspase, cyclin.
Introduction
In the developing countries of south East Asia, oral cancer is the most common cancer among males. In India, the age adjusted incidence of oral cancer ranges from 9.5/100,000 to 18.9/ 100,000 among males and 3.8/ 100,000 to 8.14/ 100,000 among females [1]. Oral cancer is also one of the common cancers that have demonstrated a strong association with tobacco habits and is often preceded by a premalignant lesion (oral leukoplakia, submucous fibrosis and others) [2]. Traditionally, oral cancer has been treated by surgery and radiotherapy either alone or in combination. Despite developments in clinical oncology and chemotherapeutics, the definitive role of chemotherapy in oral cancer is not completely defined, although a few clear indications exist. The survival from oral cancer is poor and ranges from 15% to 80% at 5 years [3 4]. The majority of advanced cancers relapse within two years of treatment [5].
Tetra-O-methyl nordihydroguaiaretic acid (M4N, currently under clinical development as EM-1421) is a novel agent that has demonstrated significant activity against Human Papilloma Virus (HPV) [6] and Human Immunodeficiency Virus (HIV) [7]. M4N has also been shown to arrest the cell cycle at the G2-M phase through inhibition of production of Cdc2 [8]. It has also been shown to induce a strong pro-apoptotic response through inhibition production and activation of survivin, resulting in down stream activation of caspase 3 and 9, with resultant cellular apoptosis. M4N is highly insoluble in water and shows little absorption when injected intra-tumoraly [8, 9, 10] as demonstrated by tritium labeling in animal models.
This study reports the clinical and molecular results of intra-tumoral injection of M4N in oral squamous carcinoma.
Patients and methods
Between November 1999 and April 2000, 21 patients were recruited. Of these, one later refused to participate, one did not complete the intended treatment and one completed M4N treatment but did not undergo scheduled surgical excision and was excluded. The final population consisted of 18 patients with a mean age of 56 years (range 34-72 years). Fourteen (77.8%) of these were males.
After obtaining written informed consent, patients were given a single 20mg (0.1mL) intralesional injection of M4N for 3 or 5 days (up to a maximum dose of 100mg total). After completing the intended M4N treatment, patients underwent scheduled surgical resection of the tumor followed by adjuvant radiotherapy if indicated. The treatment protocol employed is referred to as “treat and excise”. Toxicity was monitored using WHO toxicity criteria. A pre-treatment punch biopsy was taken to establish the diagnosis by examination of hematoxylin and eosin stained sections. Immunohistochemical (IHC) analysis of Cdc2 and survivin expression was also undertaken at this time. Post treatment surgical specimens were submitted to routine histopathological examination and IHC analysis of Cdc2 and survivin. Clinical response was recorded as presence of necrosis within the tumor after M4N injection and change in surface area of the tumor. Pathology outcome was assessed as necrosis in the post-treatment biopsy specimen and cellular apoptosis if present. The biomarker response was recorded as change in expression of Cdc2 and survivin.
Immunohistochemistry for Cdc2 and survivin
Sections of 5 μm thickness were deparaffinized in xylene and serially rehydrated in graded alcohol. Sections were treated with freshly prepared 0.3% (v/v) hydrogen peroxide in methanol in the absence of light, for 30 min, at room temperature to block the endogenous peroxidase activity. Non-specific antibody binding was then blocked using normal rabbit serum diluted 1:5 in phosphate buffered saline (PBS), for 20 min. After removal of blocking buffer primary antibody, Cdc2 (Oncogen Research Products, Cambridge, MA) or survivin (Santa Cruz Biotechnology, Santa Cruz, CA) was added to each section. The sections were then incubated overnight at 40C temperature. After washing three times with PBS, sections were incubated for 30 min with strepavidin-HRP (1:500 Vector Labs) at room temperature and were later washed three times with PBS. Sections were then incubated with peroxidase substrate solution and rinsed three more times with PBS. The immune peroxidase complexes were developed in 0.5% (v/v) 3,3’-diaminobenzidine hydrochloride (DAB; Sigma, Saint Louis, MO, USA) in PBS containing 0.03% (v/v) hydrogen peroxide. Sections were counterstained with hematoxylin and were mounted in gelatin.Scoring: Specimens were considered as "positive" for either Cdc2 or survivin if more than 5% of tumor cells within the section were positively stained. The intensity of staining was graded as mild (+), moderate (++), and intense (+++). The staining was also characterized as cytoplasmic only, nuclear only, and both cytoplasmic and nuclear with or without stromal staining.
Statistical analysis
Statistical analysis was carried out using the SPSS 13 statistical package (SPSS Inc, USA). The change in IHC staining was evaluated using the Chi-square test while survival was estimated using the Kaplan Meier method. The difference in survival for various study parameters was estimated using log-rank test.
Results
Of the 18 patients, 12 had tumors on the tongue (66.6%), 1 on buccal mucosa (5.6%) and 5 (27.8%) on the lower alveolus. Seven of the tumors were T2 (39%), 4 (22.2%) T3 and 6 (33.3%) were T4. Nine patients were node negative while 3 (16.7%) had N1 disease and 4 (22.2%) N2b disease (Table 1 ).
Untitled 1
| |
|
N (%) |
OS |
p |
DFS |
p |
EFS |
p |
| |
Survival |
|
71.7 |
- |
50.3 |
- |
49.7 |
- |
| Age |
|
|
|
|
|
|
|
|
| |
<55 |
9(50) |
100% |
0.06 |
64.2 |
0.4 |
64.2 |
0.17 |
| |
>55 |
9(50) |
35.6 |
|
36.4 |
|
25.9 |
|
| Sex |
|
|
|
|
|
|
|
|
| |
F |
4(22.2) |
75 |
0.9 |
100 |
0.18 |
75 |
0.4 |
| |
M |
14(77.8) |
51.4 |
|
38.8 |
|
32.3 |
|
| T |
|
|
|
|
|
|
|
|
| |
Tis/2 |
8(44.5) |
66.6 |
0.4 |
80 |
0.01* |
66.6 |
0.01* |
| |
Tis/2 |
10(55.5) |
44.4 |
|
17.5 |
|
15.5 |
|
| N |
|
|
|
|
|
|
|
|
| |
N0 |
9(50) |
68.5 |
0.3 |
60.9 |
0.4 |
16.9 |
0.31 |
| |
N+ |
9(50) |
43.7 |
|
33.3 |
|
29.1 |
|
| Cdc2 |
|
|
|
|
|
|
|
|
| |
Decreased |
9(50) |
70 |
0.7 |
66.6 |
0.19 |
0 |
0.17 |
| |
Others |
9(50) |
50 |
|
34.2 |
|
58.3 |
|
| Survivin |
|
50 |
|
|
|
|
|
|
Decreased |
10(55.6) |
53.3 |
0.2 |
75 |
0.01* |
53.3 |
0.15 |
| |
Others |
8(44.4) |
100 |
|
20.8 |
|
20.8 |
|
N-number of patients; OS-overall survival; DFS-disease free survival; EFS-event free survival, p-probability value; *significant
The clinical response was recorded on 5th day of drug administration and on the day of surgery. A total of 17/18 (94.4%) of the cases showed various degree of necrosis in the tumor (Figure 1, 2, 3). This ranged from minimal to extensive (+ to +++). Five of the tumors demonstrated change in surface area of the tumor (27.7%) (Figure 1). The mean reduction in surface area was 4.1 cm2 and ranged from 1.5 cm2 to 9.5 cm2. Histological necrosis was seen in 7/18 (39%) of the cases. The drug deposit in the tumor could be demonstrated in nearly all the tumors and was seen as pinkish particulate deposit.
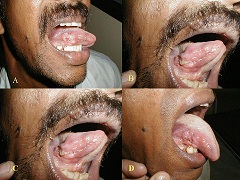
Figure 1: Clinical photograph showing necrosis A-pre treatment, B- post treatment at day 5, C-Post treatment at day 8, D- Post treatment at day 14.

Figure 2: Clinical photograph and photomicrograph showing pre treatment and post treatment changes. A-Clinical pre treatment, B- Clinical post treatment, C- pre treatment Hematoxylin and Eosin x 64, D- post treatment hematoxylin and eosin x 64, E- pre treatment Cdc2 x 64, F- post treatment Cdc2 x 64, G- pre treatment surviving x 64, H-post treatment survivin x 64

Figure 3: Clinical photograph and photomicrograph showing pre treatment and post treatment changes. A-Clinical pre treatment, B- Clinical post treatment, C- pre treatment Hematoxylin and Eosin x 64, D- post treatment hamatoxylin and eosin x 64, E- pre treatment Cdc2 x 64, F- post treatment Cdc2 x 64, G- pre treatment surviving x 64, H-post treatment survivin x 64.
Immunohistochemically, pre treatment Cdc2 and survivin expression was seen in all patients (100%). Post treatment Cdc2 inhibition was seen in 9/18 (50%) (,Figure 2 3) of the tumors while 10/18 (55.6%) showed inhibition of survivin expression (Table 1, Figure 2 3). No acute or deferred toxicities were observed in any of the patients that could be definitely attributed to M4N. No traceable quantity of drug could be identified in the blood in this study.
During the five year follow-up, 4 patients died, two from unrelated causes (one was due to a cerebrovascular event and second was due to ischemic cardiac arrest). Two patients developed a new second primary lesion (in one the new lesion was in the oropharynx and the second had multiple second primaries in the oral cavity). These two patients later died despite surgical and/or radiation salvage. Four additional patients developed recurrences in either the primary site or nodes. The overall survival was 14/18 (71.7%) (Figure 4) at 5 years while the disease free survival was 10/18 (55.6%) (Figure 5). None of the variables studied were found to influence overall survival (Table 1) while tumor stage (p=0.01) (Figure 6) and inhibition of survivin (p=0.01) (Figure 7) were found to significantly influence disease free survival. The 5 year disease free survival in patients who showed inhibition of survivin was 75% while it was 20.8% among patients who had either no change in survivin expression after the drug administration or showed an increased expression of survivin (Table 1,).
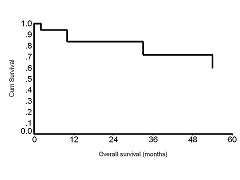

Figure 4: Overall five year survival

Figure 5: Disease free survival

Figure 6: Disease free and event free survival by tumor stage

Figure 7: Disease free survival by change in surviving expression after treatment with M4N
Discussion
The results of the present study clearly demonstrate a strong pro-apoptotic tumor cell response with clear tumor necrosis even with short exposure to M4N as shown by inhibition of Cdc2 and survivin expression in post treatment biopsy specimens. As expected, survivin inhibition significantly correlated with disease free survival, with patients who showed inhibition of survivin having significantly higher survival (75%) compared to those where there was no inhibition or where there was over expression after treatment with M4N (20.8%). Similar results related to survivin expression have been earlier observed in breast cancer [11], prostate cancer [12], ovarian cancer [13] and lung cancer [14].
Survivin has been identified as a preventer of apoptosis [15]. It is a member of the Inhibitor of Apoptosis (IAP) gene family [16]. Survivin is selectively expressed in G2-M phase and is localized to mitotic spindle microtubules [17]. In cancer, survivin has been identified as one of the important transcriptions selectively expressed in cancer cells but not in normal tissues [18, 19]. Action of M4N on survivin down-regulation is dual. During the G2-M phase, Cdc2 binds to cyclin B1 and this Cdc2-cyclin B1 complex readily phosphorylates survivin [19]. Both Cdc2 and survivin are thus prevent apoptosis [19], and it is their inhibition that produces tumor cellular apoptosis. M4N also binds to the SP1 promoter of the survivin gene, thereby decreasing surviving expression in cancer cells.[9, 10] This reduction in survivin expression allows the caspase pathway, specifically caspase 3 and 9, to bring about cellular apoptosis[9].
As M4N brings about Cdc2 down-regulation and G2-M phase arrest, the drug has potential to enhance the activity of other chemotherapeutic drugs such as paclitaxel and docetaxel if used in combination.
and docetaxel if used in combination.In conclusion the results of present study indicate that survivin is expressed in majority of oral squamous carcinoma and intralesional treatment with M4N results in survivin inhibition in most cases, thereby resulting in tumor-specific cellular apoptosis even after short exposure. Furthermore, M4N appears safe in intralesional doses of 20mg, (total dose of up to 100mg) without any acute or deferred toxicity. Inhibition of survivin by M4N appears related to increased disease free survival. However, as a single group study with no control arm, definitive conclusions about effectiveness of M4N cannot currently be made. Further randomized controlled trials of intralesional M4N either alone or in combination with Paclitaxel / Docetaxel are needed to evaluate the effect of M4N treatment in oral squamous carcinoma.
Acknowledgement
This project was supported by generous grant from Johns Hopkins University. Part of the work is supported by an academic grant from Erimos Technologies, LLC to RCH. Cost of color printing in this publication is supported by a research grant from Erimos Pharmaceutical, LLC.
The authors wish to acknowledge the help of Paragon Bioservices (Johns Hopkins Bayview Research Campus, Baltimore, MD) for preparation of the slides.
The work was presented at 58th Annual Conference of Society of Surgical Oncology held at Atlanta, USA, March 3-6, 2005; the travel of MP was supported by a travel grant from Erimos Pharmaceutical, LLC.
Contributions of Authors
MKN:Designed the study coordinated the study and helped in preparing the manuscript for publication.
MP:Helped in design and execution of the study, participated in collection of data, analysis and interpretation of data and prepared the draft manuscript.
RCH:Provided rationale and preclinical results supporting the clinical study, and carried out the biomarker assay, participated in study design and preparation of the manuscript.
All authors read and approved the final manuscript.
Competing interest
MKN:None
MP:Received an academic grant of US $5,800 from Erimos Pharmaceutical, LLC. to cover the cost of publication, namely reproduction of color photographs,
purchase of reprints and as travel support for presenting the work
RCH:Inventor of M4N. The Johns Hopkins University holds the patents to M4N and similar technologies. Erimos Technologies has licensed the patent rights from The Johns Hopkins University and is developing M4N under the designation EM-1421
Stocks: No stock in Erimos Technologies, LLC
Research grants: P-690-C25-2407 from Erimos Technologies, LLC
Other Associations: Chief Scientific Advisor to Erimos
Synopsis
Oral squamous cell carcinoma is one of the common cancers among males. This study reports on the treatment of intralesional M4N, a drug that acts by inhibition of survivin thereby causing apoptosis in oral squamous carcinoma
References
[1]Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, eds Cancer Incidence in Five Continents, Vol. VIII, IARC Scientific Publications No. 155, Lyon, IARC 2002.[PubMed]
[2].Nair MK, Sankaranarayanan R, Padmanabhan TK, Padmakumary G. Clinical profile of 2007 oral cancers in Kerala, India. Ann Dent 1988; 47: 23-26.[PubMed]
[3].Kosary GL, Ries LAG, Miller BA, Hankey BF, Harras A, Edwards BK, editors. SEER Cancer Statistics Review, 1973-1992: tables and graphs. NIH Pub. No. 96-2789. Bethesda:National Cancer Institute, 1995.
[4].Sankaranarayanan R, Black RJ, Parkin DM. Cancer survival in developing countries. IARC Scientific Publication No. 145. Lyon: International Agency for Research on Cancer, 1998.[PubMed]
[5].Sankarnarayanan R, Mathew B, Jacob BJ, Thomas G, Somanathan T, Pisani P et al. Early findings from a community based cluster randomized controlled oral cancer screening trial in Kerala, India. Cancer 2000; 88: 664-73.[PubMed]
[6]Craigo J, Callahan M, Huang RC, DeLucia A. Inhibition of Human Papilloma virus type 16 gene expression by nordihydroguaiaretic acid plant lignan derivatives. Antiviral Research 2000; 47: 19-28.[Pubmed]
[7]Gnabre JN, Brady JN, Clanton DJ, Ito Y, Dittmer J, Bates R, Huang R. Inhibition of human immunodeficiency virus type 1 transcription and replication by DNA sequence-selective plant lignans Proc Nat Acad Sci 1995; 92: 11239-11243.[Pubmed] [PMC Full Text]
[8]Heller JD, Kuo J, Wu W, Kast M, Huang RCC. Tetra-o-methyl nordihydroguaiaretic acid induces G2 Arrest in mammalian cells and exhibits tumoricidal activity in vivo. Cancer Res 2001; 61: 5499-5504 [Pubmed] [Free Full Text]
[9]Chang C, Heller JD, Kuo J, Huang RCC. Tetra-o-methyl nordihydroguaiaretic acid induces growth arrest and cellular apoptosis by inhibiting Cdc2 and survivin expression. Pro Natl Acad Sci 2004; 101: 13239-13244. [Pubmed] [Free Full Text]
[10Park R, Chang C, Liang Y, Chung Y, Henry RA, Lin E, Mold DE, Huang RCC. Systemic treatment with Tetra-O-methyl nordihydroguaiaretic acid suppresses the growth of human xenograft tumors. J Clin cancer Res 2005 ;11:4601-4609.[PubMed] [Free Full Text]
[11]Chu JS, Shew JY, Huang CS. Immunohistochemical analysis of survivin expression in primary breast cancers. J Formos Med Assoc 2004;103:925-31.[PubMed]
[12].Kishi H, Igawa M, Kikuno N, Yoshino T, Urakami S, Shiina H. Expression of the survivin gene in prostate cancer: correlation with clinicopathological characteristics, proliferative activity and apoptosis. J Urol 2004; 171:1855-60.[Pubmed]
[13]CVischioni B, van der Valk P, Span SW, Kruyt FA, Rodriguez JA, Giaccone G. Nuclear localization of survivin is a positive prognostic factor for survival in advanced non-small-cell lung cancer. Ann Oncol 2004; 15: 1654-60.[Pubmed]
[14]Holmann HS, Simm A, Hammer A, Silber RE, Bartlling B. Expression of inhibitors of apoptosis (IAP) proteins in non-small cell lung cancer. J cancer Res Clin Oncol 2002; 128: 554-60.[Pubmed]
[15]Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 1997; 3: 917-21. [PubMed]
[16].Deveraux QL, Reed JC. IAP family proteins--suppressors of apoptosis. Genes Dev 1999; 13: 239-52.[PubMed] [Free Full Text]
[17].Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998; 396: 580-84.[Pubmed]
[18].Velculescu VE, Madden SL, Zhang L, Lash AE, Yu J, Rago C, Lal A, Wang CJ, Beaudry GA, Ciriello KM, Cook BP, Dufault MR, Ferguson AT, Gao Y, He TC, Hermeking H, Hiraldo SK, Hwang PM, Lopez MA, Luderer HF, Mathews B, Petroziello JM, Polyak K, Zawel L, Kinzler KW, et al. Analysis of human transcriptomes. Nat Genet 1999; 23:387-88.[pubMed]
[19].O’Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, Tognin S, Marchisio PC, Altieri DC. Regulation of apoptosis at cell division by p34 Cdc2 phosphgrylation of survivin. Proc Natl Acad Sci 2000; 97:13103-13107.[Pubmed]

