Research

Importance of bcl2 protein expression in histological negative margins after excision of oral squamous cell carcinoma
1Devendra Kumar Ravi, 2Mohan Kumar,3Mridula Shukla,
1Manoj Pandey,
- 1Department of surgical oncology, Banaras Hindu
university Varanasi India
- 2Department of Pathology, Banaras Hindu
university Varanasi India
- SRL Diagnostics, Varanasi
- Submitted: January 7, 2017
- Accepted: February 14, 2017
- Published: February 14, 2017
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Background
B cell lymphoma-2 (bcl-2) is an intercellular membrane associated protein that functions as anti apoptotic protein via mitochondria mediated intrinsic pathway of caspase activation. We evaluated expression of bcl2 protein in patients with oral cancer and in adjacent normal tissue.
Patients and methods
Bcl-2 protein expression was evaluated in patients with oral cancer undergoing surgical resection using immunohistochemistry (IHC) in the tumour tissue and the adjacent normal tissue.
Results
Bcl2 protein expression was not seen in any tumour tissue however, in the normal margin 61 cases showed bcl2 expression. This expression was cytoplasmic in all the cases.
Conclusions
This expression in margins correlated with lymphatic spread of the disease and suggest poor prognosis in this subset of the patients. The mRNA expression, polymorphism, mutations, SNP’s were not looked at in the present study and hence, the exact significance of the results is not clear.
Key words
apoptosis; oral cancer; tongue; buccal mucosa; alveolus; lymph node
Introduction
B-cell lymphoma -2 (Bcl-2) is the second member of a range of proteins first discovered as the reciprocal gene translocation in chromosomes 14 and 18 locus in B-cell leukaemia. It is an intracellular membrane-associated protein that functions to block programmed cell death. Main role of Bcl-2 is in regulating a major apoptotic pathway [1]. This is the mitochondria-mediated or intrinsic pathway of caspase activation. The site of action for the Bcl-2 family is mostly on the outer mitochondrial membrane. Within the mitochondria are apoptogenic factors that if released activate the executioners of apoptosis, i.e. the caspases. Depending on their function, once activated, Bcl-2 proteins either promote the release of these factors, or keep them sequestered in the mitochondria [2].
Bcl-2-regulate the pathway by controlling three major subgroups and the five pro-survival members, i.e. Bcl-2, Bcl-xL, Mcl-1, Bcl-w & A1. These are required for cell survival [3].
antiproliferative by facilitating G0 phase of the cell cycle while BAX is proapoptotic and accelerates S-phase progression [3]. When pro-apoptotic family members such as Bax and Bak are activated (Figure 1) then pores in the outer membrane of the mitochondria opens, allowing the release of proteins that initiate apoptosis [4, 5]. Bcl-2 is a tumorigenic protein that is expressed in cancers. It is elevated in many cancers, including breast, colon, prostate, small cell lung cancer, chronic lymphocytic leukaemia and low-grade lymphomas .
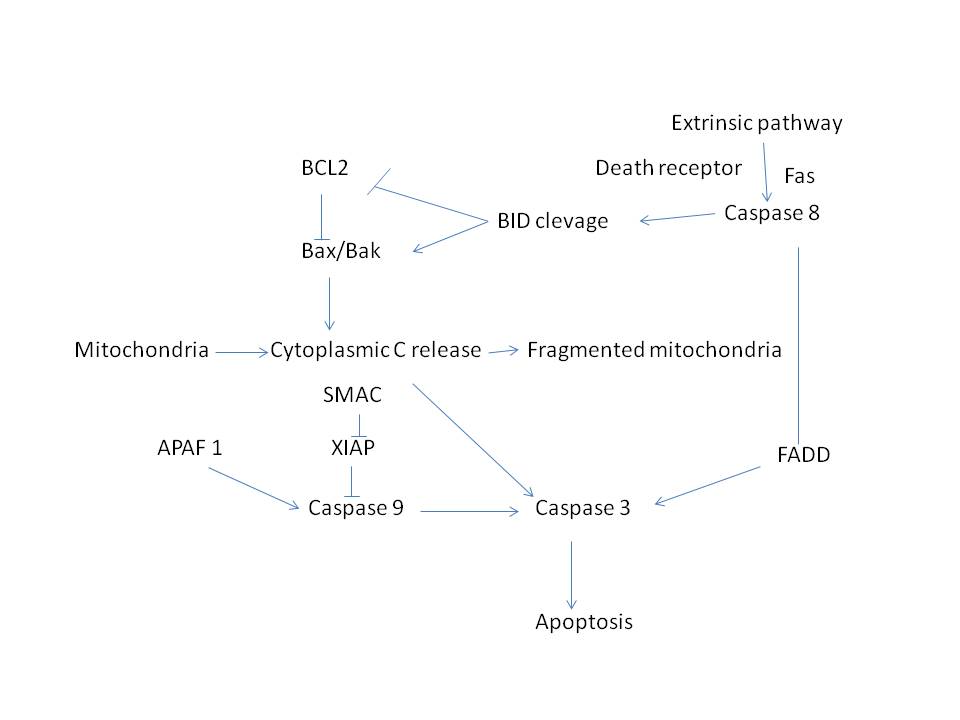
Figure 1: BCL2 apoptosis pathway and its regulation by intrinsic and extrinsic pathway
Bcl-2 expression in oral cancer has been reported in 50% to 75% of cases. In normal oral mucosa, Bcl-2 is not detectable or is expressed only occasionally in the basal cells [6]. Teni T et al.[7], found over expression of tumour specific cytoplasmic Bcl-2 in 56% and bax in 43% oral cancers. Expression of Bcl-2 was seen in 16% premalignant oral lesions comprising leukoplakia’s and submucous fibrosis while expression of Bax was seen in 55% [7].
Unlike lymphoma, in the oral cancer, the reason for over expression is unknown because there is no genetic rearrangement. More so, over expression of Bcl-2 is not a universal feature of cancer as it is not seen in malignant melanomas. However, when expression of Bcl-2 is inhibited, some cancer cells lose their malignant behaviour. Bcl2 over expression has been studied in the head neck cancer and pre cancer [2, 6, 8-12]. It has been found to dysregulate cell-cycle, predict the response to chemotherapy and has also been found to be a useful prognostic marker. We carried out this study to look at the expression profile of bcl2 in oral cancers and correlate it with pathological markers.
Patients and Methods
This study was carried out in the Department of Surgical Oncology and Department of Pathology, Institute of Medical Sciences Banaras Hindu Varanasi between 2008 and 2012 with the approval of the Institute Ethics Committee. After obtaining written informed consent, tumour specimens from 100 patients oral Squamous cell carcinoma (OSCC) were collected along with the tissue from the margins at least 1 cm away from the primary tumours. Only patients diagnosed with primary OSCC without any prior treatment, where surgery as primary curative treatment was performed were included. Histological negative surgical margins were available from all 100 patients. Bcl-2 protein expression was determined by immunohistochemistry. The expression of Bcl-2 was also correlated with the grade of tumour, pathological lymph node metastasis and other findings in the specimens.
Immunohistochemistry
Clean glass slides with 95% ethanol were treated with 1% poly-L-Lysin solution and were air dried. 4 micron thick tissue sections were cut using microtome and slides were placed in incubator at 60oC for one hour. All slides were deparaffinize using 3x- Xylene for 5 minutes each , 2x- 100% ethanol for 5minutes each , 2x- 95% ethanol 5 minute respectively . Slides were washed and excess liquid was drained. Antigen retrieval was done in sodium citrate buffer (pH 6.0) solution and heated the slides at 95oC for 10 minute in microwave oven.
To quench endogenous peroxidase activity, slides were incubated for 5 minutes in 1-3 drops of peroxidase block. Rinsed with PBS and were transferred to a PBS wash for 2 minute on stir plate. Thereafter the slides were incubated for 20 minutes in 1-3 drops serum block, and Primary antibody in 1: 200µl dilution was applied. Thereafter the slides were incubated over night at 40oC. 1-3 drops secondary antibody was applied and slides were incubated for 30 minutes. This was followed by incubation for 30 minutes in 1-3 drops HRP- Streptavidin complex. After the washing 1-3 drops of HRP substrate was added to each slide.
Counterstaining of slides was done with haematoxylin, followed by dehydration with 2x 95% ethanol for 10 second each , and 2x 100% ethanol for 10 second each and 3x xylenes for 10 seconds each and were mounted.
Grading of the bcl2 staining:
All slides observed by light microscope and staining was graded as follows:- (i) Positive (ii) Negative (iii) Equivocal.
Beside the following were also recorded for each slide; type of staining :- (i) Cytoplasmic (ii) Nuclear (iii) Membranous and staining was graded as: (i) 1+(<25%) (ii) 2+ (25-50%) (iii) 3+ (51-75%) and (iv) 4+ (>75% cells stained).
Statistical analysis
The data is presented as categorical variables and the statistical analysis was done by Chi Square test.
Results
Of the 100 patients, 44 were of cancer of the buccal mucosa, 12 of tongue, 19 lower alveolus, 14 lip, and 11 gingivo-buccal complex. Bcl2 protein expression was not seen in any tumour tissue (Figure 2) however, in the normal margin 61 cases showed bcl2 expression. This expression was cytoplasmic in all the cases. Of these, 29 were of buccal mucosa, 7 tongue, 11 alveolus, 6 GB sulcus and 8 cases were of lip. In 30 of these the tumours were well differentiated, 24 moderate differentiation and 7 were poorly differentiated. The bcl2 staining was seen only in cases with lymph node metastasis (61/80), the 20 patients without lymphatic spread did not show any positivity for bcl2 staining. This difference was statistically significant. The detail of the expression profile is given in Table 1. Of the 61 cases, the 15 cases showed 1+, 20 showed 2+, 18 showed 3+, and 8 showed 4+ cytoplasmic positivity.
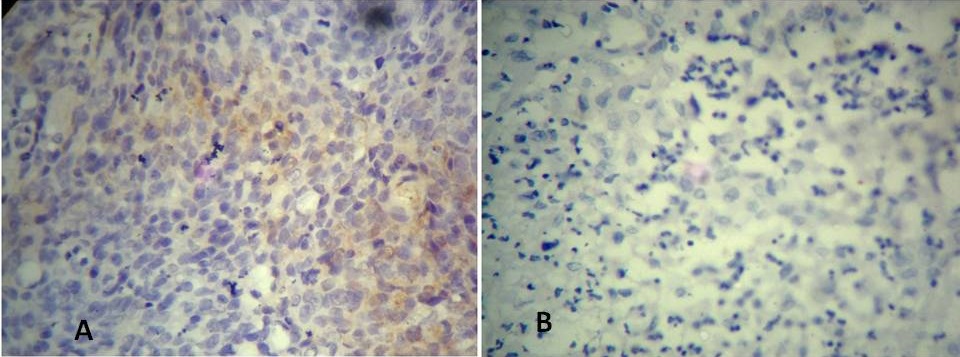
Figure 2: Photomicrograph showing negative BCL2 staining in (A) Grade III tumour and (B) grade II tumour.
| Variable |
Bcl-2 Positive Tumor Samples |
Bcl-2 Negative Tumor Samples |
Bcl Positive Adjacent Tisue Samples |
Bcl-2 Nagative Adjacent Tissue Samples
|
P value |
| Overall-100 |
| |
00 |
100 |
61 |
39 |
- |
| Staining |
|
|
|
|
|
Cytoplasmic 00
|
-- |
Cytoplasmic 61
|
100 |
| Nuclear-00 |
-- |
Nuclear-00 |
-- |
| Membranous-00 |
-- |
Membranous- |
-- |
| Scoring |
+ =00 |
-- |
+ =15 |
-- |
| 2+ =00 |
-- |
2+ =20 |
-- |
| 3+ =00 |
-- |
3+ =18 |
-- |
| |
|
|
|
| Degree of differentiation |
Well differentiated
|
00 |
100 |
30 |
50 |
.761589 |
Moderately differentiated
|
00 |
24 |
35 |
Poorly differentiated
|
00 |
07 |
15 |
| Lymph Node Status |
Lymph node positive
|
00 |
80 |
P value = 1.0000 ( It is not
statistically significant ) |
61 |
19 |
<0.0001 |
Lymph node
negative
|
00 |
20 |
00 |
20 |
| Sites |
| Name of Site |
Postive Cases |
Negative Cases |
Total cases |
Positive
Cases
|
Negative Cases |
Total
cases
|
.933174 |
| Buccal Mucosa |
00 |
44 |
44 |
29 |
15 |
44 |
| Tongue |
00 |
12 |
12 |
7 |
5 |
12 |
| Alveolus |
00 |
19 |
19 |
11 |
18 |
19 |
| Lip |
00 |
14 |
14 |
8 |
6 |
14 |
| GB sulcus |
00 |
11 |
11 |
6 |
5 |
11 |
| Total Sites |
00 |
100 |
100 |
61 |
39 |
100 |
Discussion
The Bcl2 family proteins comprise the sentinel network that regulate the mitochondrial or intrinsic apoptotic response [13]. The bcl2 expression in the cancers of the head neck including oral cavity has been studied [2, 8-10, 14-21]. The expression is seen in up to 40% of the cases and higher expressions have been reported in premalignant lesions like leukoplakia and submucous fibrosis. In the present study however, none of the tumour showed positivity for bcl2, while 61% of the normal margin tissue showed bcl2 expression. The expression was significantly found to correlate with aggressive disease i.e. patients who were cervical lymph nodes positive compared to those who were negative suggesting that bcl2 expression in the margins can be used as a marker of aggressive disease. Earlier studies too has found bcl2/bax ratio as predictor of aggressive disease [14].
Due to the sharing of the same microenvironment, adjacent normal tissue also may show molecular similarity to tumour tissues [22, 23], it has been shown that the adjacent tissue may produce growth factors and may also help modify the matrix thereby modifying the disease progression. Again in these situations expression of bcl2 and alteration of bcl2/bax ratio may play a very significant role [23].
The expression has also been correlated with the response to chemotherapy, radiotherapy and presence of human papilloma virus [8, 18, 19, 22, 24, 25]. Deng et al., showed lowering of expression in malignant tissues compared to that in normal tissues [21], similar to ours where malignant tissue showed no expression at all. Studies of Teni et al., [7] has suggested that bcl2 expression is an early even in oral carcinogenesis and hence, it is possible that in our cases expression was not seen as most cases were advanced. Crowe et al., showed that the response of p53 is mediated by bcl2 in oral cancer cells [26]. Similarly, expression of bcl2 has been found to increase after vitamin A therapy suggesting its potential role in carcinogenesis [20]. Studies have also looked at the bcl2 pleomorphism and BCL2 ala43ala genotype is found to be at increased risk for developing tumours [27], however BCL2 (-938 C>A) failed to show any association with carcinogenesis [28], but on the other had was found to effect overall and relapse free survival [12]. A few studies have also looked at bcl2 expression and human palilloma virus and have found poor prognosis in HPV +/BCL2- tumours [18]. A meta analysis on biological markers in oral cancer showed some improvement in survival in patients expressing bcl2, however, due to smaller studies and in absence of enough power no significance can be attached to these results [29].
Conclusions
Results of the present study show that the tumours did not express bcl2 while 61% of the healthy margins expressed bcl2. This expression in margins correlated with lymphatic spread of the disease and suggest poor prognosis in this subset of the patients. The mRNA expression, polymorphism, mutations, SNP’s were not looked at in the present study and hence, the exact significance of the results is not clear. Further studies incorporating all aspects and up and downstream signalling are needed for definite conclusions.
Authors’ contribution
DKR- literature search, experiment and data collection
MK- Data collection, design and interpretation of results
MS- Interpretation of results and preparation of manuscript
MP- design of the study, conceiving of the idea and preparation of manuscript.
Ethical considerations
The study was approved by the Institute ethics committee
Funding
None
Reference List
[1].Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2002 September;2(9):647-56.[PubMed]
[2].Rahman MA, Amin AR, Wang D et al. RRM2 regulates Bcl-2 in head and neck and lung cancers: a potential target for cancer therapy. Clin Cancer Res 2013 July 1;19(13):3416-28. [PubMed]
[3].Zinkel S, Gross A, Yang E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ 2006 August;13(8):1351-9. [PubMed]
[4].Karch J, Kwong JQ, Burr AR et al. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. eLife 2013 August 27;2:e00772. [PubMed]
[5].ang C, Youle RJ. The Role of Mitochondria in Apoptosis(). Annu Rev Genet 2009;43:95-118. [PubMed]
[6].Sudha VM, Hemavathy S. Role of bcl-2 oncoprotein in oral potentially malignant disorders and squamous cell carcinoma: an immunohistochemical study. Indian J Dent Res 2011 July;22(4):520-5. [PubMed]
[7].Teni T, Pawar S, Sanghvi V, Saranath D. Expression of bcl-2 and bax in chewing tobacco-induced oral cancers and oral lesions from India. Pathol Oncol Res 2002;8(2):109-14. [PubMed]
[8].Moreno-Galindo C, Hermsen M, Garcia-Pedrero JM, Fresno MF, Suarez C, Rodrigo JP. p27 and BCL2 expression predicts response to chemotherapy in head and neck squamous cell carcinomas. Oral Oncol 2014 February;50(2):128-34. [PubMed]
[9].Geomela PA, Kontos CK, Yiotakis I, Scorilas A. Quantitative expression analysis of the apoptosis-related gene, BCL2L12, in head and neck squamous cell carcinoma. J Oral Pathol Med 2013 February;42(2):154-61 [PubMed]
[10].Fendri A, Kontos CK, Khabir A, Mokdad-Gargouri R, Scorilas A. BCL2L12 is a novel biomarker for the prediction of short-term relapse in nasopharyngeal carcinoma. Mol Med 2011 March;17(3-4):163-71. [PubMed]
[11].Fendri A, Kontos CK, Khabir A, Mokdad-Gargouri R, Ardavanis A, Scorilas A. Quantitative analysis of BCL2 mRNA expression in nasopharyngeal carcinoma: an unfavorable and independent prognostic factor. Tumour Biol 2010 October;31(5):391-9. [PubMed]
[12].Lehnerdt GF, Franz P, Bankfalvi A et al. The regulatory BCL2 promoter polymorphism (-938C>A) is associated with relapse and survival of patients with oropharyngeal squamous cell carcinoma. Ann Oncol 2009 June;20(6):1094-9. [PubMed]
[13].Hata AN, Engelman JA, Faber AC. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov 2015 May;5(5):475-87. [PubMed]
[14].Giotakis AI, Kontos CK, Manolopoulos LD, Sismanis A, Konstadoulakis MM, Scorilas A. High BAX/BCL2 mRNA ratio predicts favorable prognosis in laryngeal squamous cell carcinoma, particularly in patients with negative lymph nodes at the time of diagnosis. Clin Biochem 2016 August;49(12):890-6 [PubMed]
[15].Lehnerdt GF, Bachmann HS, Adamzik M et al. AQP1, AQP5, Bcl-2 and p16 in pharyngeal squamous cell carcinoma. J Laryngol Otol 2015 June;129(6):580-6. [PubMed]
[16].Knopf A, Lempart J, Bas M, Slotta-Huspenina J, Mansour N, Fritsche MK. Oncogenes and tumor suppressor genes in squamous cell carcinoma of the tongue in young patients. Oncotarget 2015 February;6(5):3443-51. [PubMed]
[17].Coutinho-Camillo CM, Lourenco SV, Nishimoto IN, Kowalski LP, Soares FA. Expression of Bcl-2 family proteins and association with clinicopathological characteristics of oral squamous cell carcinoma. Histopathology 2010 August;57(2):304-16. [PubMed]
[18].Nichols AC, Finkelstein DM, Faquin WC et al. Bcl2 and human papilloma virus 16 as predictors of outcome following concurrent chemoradiation for advanced oropharyngeal cancer. Clin Cancer Res 2010 April 1;16(7):2138-46.
[19].Csuka O, Remenar E, Koronczay K, Doleschall Z, Nemeth G. Predictive value of p53, Bcl2 and bax in the radiotherapy of head and neck cancer. Pathol Oncol Res 1997 September;3(3):204-10. [PubMed]
[20].Varma D, Gupta S, Mandal AK. Role of p53 and bcl2 as markers of vitamin A response in premalignant lesions of the oral cavity. Indian J Pathol Microbiol 2007 January;50(1):15-7. [PubMed]
[21].Deng ZY, Wang YH, Quan HZ et al. Investigation of the association between miR181b, Bcl2 and LRIG1 in oral verrucous carcinoma. Mol Med Rep 2016 October;14(4):2991-6. [PubMed]
[22].Heise T, Kota V, Brock A et al. The La protein counteracts cisplatin-induced cell death by stimulating protein synthesis of anti-apoptotic factor Bcl2. Oncotarget 2016 May 17;7(20):29664-76. [PubMed]
[23].Raudenska M, Sztalmachova M, Gumulec J et al. Prognostic significance of the tumour-adjacent tissue in head and neck cancers. Tumour Biol 2015 December;36(12):9929-39. [PubMed]
[24].Sarkar S, Maiti GP, Jha J et al. Reduction of proliferation and induction of apoptosis are associated with shrinkage of head and neck squamous cell carcinoma due to neoadjuvant chemotherapy. Asian Pac J Cancer Prev 2013;14(11):6419-25. [PubMed]
[25].Crowe DL, Sinha UK. p53 apoptotic response to DNA damage dependent on bcl2 but not bax in head and neck squamous cell carcinoma lines. Head Neck 2006 January;28(1):15-23. [PubMed]
[26].Jain M, Kumar S, Lal P, Tiwari A, Ghoshal UC, Mittal B. Role of BCL2 (ala43thr), CCND1 (G870A) and FAS (A-670G) polymorphisms in modulating the risk of developing esophageal cancer. Cancer Detect Prev 2007;31(3):225-32. [PubMed]
[27].Chen K, Hu Z, Wang LE et al. Single-nucleotide polymorphisms at the TP53-binding or responsive promoter regions of BAX and BCL2 genes and risk of squamous cell carcinoma of the head and neck. Carcinogenesis 2007 September;28(9):2008-12. [PubMed]
[28].Rainsbury JW, Ahmed W, Williams HK, Roberts S, Paleri V, Mehanna H. Prognostic biomarkers of survival in oropharyngeal squamous cell carcinoma: systematic review and meta-analysis. Head Neck 2013 July;35(7):1048-55. [PubMed]
[29].Rainsbury JW, Ahmed W, Williams HK, Roberts S, Paleri V, Mehanna H. Prognostic biomarkers of survival in oropharyngeal squamous cell carcinoma: systematic review and meta-analysis. Head Neck 2013 July;35(7):1048-55. [PubMed]

