Original Article

Study of Immunohistochemistry and Aggressiveness of Non-functioning Pituitary Adenomas (Implication on Management): A Series of 30 Cases.
1 Manash Bora, 1Radhey Shyam Mittal, 2 Shashi Singhvi, 1Achal Sharma
- 1Department of Neurosurgery, SMS Medical College, Jaipur, India.
- 22Patho Care &Research Centre, A-34, Prabhu Marg, opposite A C Market, Raja Park, Jaipur, India
- Submitted September 22, 2014
- Accepted:April 10, 2015
- Published:April 26, 2015
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Introduction
Pituitary adenomas are the third most common brain tumor comprising 10-20% of primary brain tumors and nearly 25% are non functioning pituitary adenoma (NFPA). NFPA are larger tumors and many of them are invasive and tend to recur or regrow. Regarding management of NFPA there is no clear common consensus for indication of adjuvant therapies.
Study Design
A prospective study of clinically non-functioning pituitary adenoma cases operated in our institute from June 2013 to May 2014 were done.
Patients and Methods
Clinical data of patients, biochemistry, radiology, per-operative findings, histopathology, and immunohistochemistry(IHC) with proliferative index KI-67/MIB are analyzed and their correlation with biological aggressiveness is searched for.
Results
Total 30 cases were studied. Majority of them are found to have cells positive for LH/FSH (36.66%), and prolactin (PRL) (16.66%), ACTH (6.66%), GH (6.66%), Pleurihormonal (23.33%). In 10% tumors no hormone cell was positive hence termed Null cells. Radiological invasiveness was found in66.66% (20/30) cases and 30% of them had high KI-67.
Conclusion
KI-67/MiB and its relation with invasiveness of tumor, certain HPE type & Hormone markers may be key in understanding biological behavior of NFPAs &selection of patients for strict follow-up and adjuvant radiotherapy.
Introduction
Pituitary adenomas are the third most common brain tumor comprising 10-20% of primary brain tumors and nearly 25% are NFPA. By and large, NFPA are larger tumors and many of them are invasive and tend to recur or regrow [1, 2]; as total resection of tumor is not always possible due to involvement of vital structures like optic apparatus, major vessel like internal carotid artery and hypothalamus. Regarding management of NFPA there is no clear common consensus for indication of adjuvant therapies. Adjuvant medical therapies like dopamine receptor agonists do not have any promising role [3, 4]. Postoperative radiotherapy (RT) is effective in selected cases [1, 5, 6, 7, 8]. Not all residual tumors regrow even when no RT is offered. Postoperative RT carries high chance of developing pan-hypopituitarism rendering patient hormone dependent and also affecting life expectancy [9]. There are reports of cardiovascular complication and sudden death due to hypopuitarism [10]. In addition, there are risks of neurocognitive impairment, development of radiation induced carcinomas, radiation damage of optic apparatus following RT for Pituitary tumors [2, 11, 12, 13, 14, 15, 16]. Considering the potential side effects and difficulty in selection of patients for postoperative RT, the usefulness of postoperative radiotherapy is still controversial. Selecting patients for RT is difficult as there are no specific markers of tumor aggressiveness. We studied IHC markers of NFPA along with KI-67/MIB index as marker of proliferation and its correlation with biological aggressiveness.KI-67 is the most widely accepted marker of proliferation [17, 18, 19, 20].
Patients and Methods
Clinically and biochemically non-functioning pituitary adenoma cases operated in our institute from June 2013 to May 2014 were included in the study. Patients with either clinical or biochemical evidence of pituitary hyperfunction were excluded.
Clinical data, biochemistry, radiology, per-operative findings, histopathological examination (HPE), IHC with proliferative index were analyzed.
CT scan brain and sella with contrast and contrast MRI brain/sella were done to determine the tumor size, suprasellar and para-sellar extension and bony changes of sellar floor. These imaging studies were used to define invasiveness; extension beyond sella was termed to be invasive.
HPE was done, histologic pattern of the tumor were identified. Histopathological examination with particular importance to pattern like papillary, pseudopapillary, trabecular, acinar, diffusely arranged, sinusoidal etc. were detected and their correlation with proliferative markers were analyzed
Immunohistochemistry done for hormonal markers for LH/FSH, ACTH, hGH, TSH, hProlactin along with synaptophysin and proliferative marker KI-67/MIB by using Biogenex ready-to-use test kit after antigen retrieval. Antigen retrieval was done by retriever and using appropriate buffering agent. Correlation of KI-67/MiB with various IHC markers, histological pattern and aggressiveness in terms of radiological & clinical behavior were looked for.
Patients were followed up with contrast MRI sella at immediate post-operative, at 6 months post-operative and at 18 months post-op. Amount of any residual tumor and recurrence or increase in tumor size were identified on an average follow up of one year (maximum 19 months and minimum 6 months of follow-up).
Results
Total 30 cases of NFPA are studied and IHC findings are like given in (Table 1).Most cases were found between 30-50 years of age, only 1 case was found below20 years and 1 case above 60 years of age (Figure 1). Female sex was predominant (Figure 2).
|
Hormone markers
|
LH/FSH
|
PRL
|
ACTH
|
GH
|
Pleuri Hormonal
|
NullCell
|
|
No of cases
(% of total)
|
11/30
(36.66%)
|
5/30
(16.66%)
|
2/30
(6.66%)
|
2
/30
(6.66%)
|
7/30
(23.33%)
|
3/30
(10%)
|
|
KI-67=0-1%
|
6
|
0
|
0
|
1
|
7
|
2
|
|
KI-67=1-3%
|
4
|
2
|
1
|
1
|
0
|
1
|
|
KI-67>3%
|
1
|
3
|
1
|
0
|
0
|
0
|
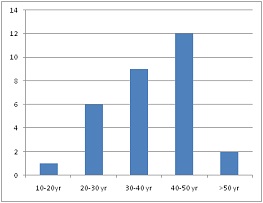
Figure 1: Age distribution
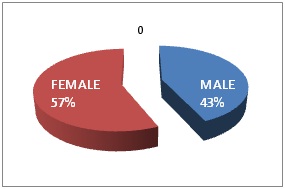
Figure 2: Sex distribution
Twenty percent patients having high KI-67/MIB-1 value above 3%. Tumors having prolactin and ACTH secreting cells were found to have higher KI-67 value (Table1). Radiological invasiveness, as determined by MRI/CT brain & sella, were found in 16 out of 30 cases; 6 out of 16 had high KI-67 (37.50%) and 14 out of 16 (87.50%) had KI-67 value>1% (Table 3).
Cases confined to sella mostly had KI-67<1%. This shows that tumors with high KI-67 have higher chance of being invasive. Histopathological patterns were analyzed and their relation with proliferative index was searched (Table 2) and it was seen that diffusely arranged and pseudopapillary patterns had high KI-67. Sinusoidal pattern showed high as well as low KI-67.Gonadotrophins (LH/FSH) constituted the majority (36.66%) of hormonal markers (Figure 3).
|
HPE type
|
Pseudopapillary
|
Sinusoidal
|
Trabecular
|
Acinar
|
Diffusely Arranged
|
Amyloid
|
Apoplexy
|
papillary
|
|
No of Patients
|
10
|
2
|
2
|
2
|
10
|
1
|
1
|
2
|
|
KI-67 value
|
4=3-5%
6=1-3%
|
1=3-5%
1=1-3%
|
0-1%
all
|
0-1%
all
|
4=0-1%
5=1-3%
1=10-15%
|
0-1%
all
|
0-1%
all
|
0-1%
all
|
|
KI-67 Value
|
No of Patients
|
Radiological invasiveness
|
|
Positive
|
Negative
|
|
0-1%
|
12
|
2
|
10
|
|
1-3%
|
12
|
8
|
4
|
|
3-5%
|
5
|
5
|
0
|
|
>5%
|
1
|
1
|
0
|
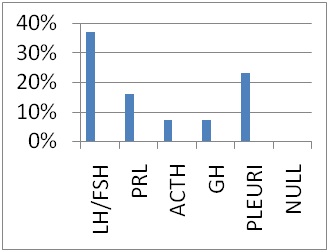
Figure 3: Hormone Positivity
Discussion
Various case series show different views regarding correlation of KI-67 with invasiveness of pituitary adenomas. Shibuya
et al., studied 65 cases of pituitary adenomas and their KI-67 values and found high values of KI-67 in recurrent, nonfunctioning and ACTH secreting adenomas [21]. In our study we also found similar results.
Ekramullah et al., found that mean KI-67 index was higher among recurrent adenomas. They studied 14 regrowing NFPA and 19 cases without recurrence/regrowth and found that KI-67 index was higher in the regrowing group and this difference was statistically significant [22]. In our series we got significantly high KI-67 value in recurrent cases. Thapar
et al., studied 70 cases of pituitary adenomas and seven carcinomas and found that mean KI-67 index was 1.37% in non-invasive, 4.66% in invasive and 11.9% among carcinomas [23]. Our series showed that 87% of invasive cases have KI-67>1% and 37% of invasive cases have KI-67>3%. Asano
et al., studied 63 cases of pituitary adenomas and found high KI-67 values ranging 2-6% in prolactin secreting tumors and low values in GH secreting tumors, these tumors were functional tumors [17].
Landolt et al., studied 31 cases of pituitary adenomas and found KI-67 values between 1-3.7% in ACTH secreting and invasive tumors [24]. In our study we found that among tumors with high KI-67 value; 50% have prolactin secreting cells and 20% have ACTH secreting cells and 20% have gonadotrophin secreting cells as identified by IHC. Tumors having GH secreting cells and Pleurihormonal cells are showing low KI-67 value.L Mastronardi
et al., after analyzing 103 cases suggested that tumors with high KI-67 value are destined for post-op relapse [18]. Knosp
et al., reported higher Ki-67 labelling index (p< 0.05) in histologically invasive adenomas [25]. Daita
et al.,observed a higher MIB-1 positive ratio withdural invasion than with non-invasive tumors(p< 0.05) [20]. However, other authors failed to find any significant correlation between KI-67 and tumor invasiveness, recurrence or hormone types [26, 27, 28].
Wolfsberger et al., considered KI-67 as gold standard to assess proliferation in NFPA [29]. Rishi
et al., studied 77 NFPA and observed low KI-67/MIB-1 in NFPA and found no relation bet age sex or duration of symptoms [30]. While going through the literature we could not find any study which analyzed proliferative index KI-67 in NFPA and its correlation in terms of invasiveness, hormone cells present and histopathologic pattern. That’s how this study is unique.
As found by many authors we also found a strong correlation between invasive tumors and high KI-67. We found that tumors having Prolactin & ACTH secreting cells were showing high proliferative index which was earlier observed in similar functional tumors [24]. Invasive tumors were found to have high KI-67 value which is supported by studies of many authors [17, 18, 20, 21, 22, 23, 24 and 25]. In our series we found very high KI-67 value in recurrent tumor (10-15%). Also we studied and found that HPE types like “diffusely arranged” and “pseudopapillary” pattern were showing high KI-67 in most cases. So we put these types of HPE in “alarming zone” and patients are advised post-op radiotherapy and stricter follow-up. “Sinusoidal” type showed mixed value and were put in grey zone.s
Conclusion
These correlations of proliferative index may be an important determining factor in selecting patients for post-operative RT i.e. offering RT to patients of high proliferative index and avoiding potential adverse effects of RT in patients of low proliferative index. Predicting recurrence and selecting patients for strict follow-up may also be possible.
Learning points
•High KI-67/MIB-1 index in pituitary tumors predicts increased chance of recurrence.
•Non-functioning pituitary tumors with ACTH and Prolactin markers in immunohistochemistry have higher predilection for recurrence
•Patients having tumors with high proliferative index or high chance of recurrence are good candidates for post-operative radiotherapy.
• Patients having tumors with high proliferative index or high chance of recurrence are to be followed strictly at frequent intervals.
Authors’ contributions
MB: Conceived and designed the study and prepared the manuscript.
RSM: Conceived and designed the study and prepared the manuscript.
SS: Conceived and designed the study and prepared the manuscript.
AS: Conceived and designed the study and prepared the manuscript.
Ethical Considerations
The study was approved by the institute ethics committee and written informed consent was taken from all subjects participating in the study.
Conflict of interests
The authors declare that there are no conflicts of interests to declare
Funding
None
References
[1Turner HE, Stratton IM, Byrne JV, Adams CB,Wass JA. Audit of selected patients with nonfunctioning pituitary adenomas treated without irradiation – a follow-up study. ClinEndocrinol (oxf) 1999; 51: 281–284. [PMID: 10469006][Pubmed]
[2]Boelaert K,Gittoes NJ. Radiotherapy for non-functioning pituitary adenomas. Eur J Endocrinol 2001; 144: 569–575. [PMID: 11375790 [Pubmed]
[3]de Bruin TW, Kwekkeboom DJ, Van’tVerlaat JW, Reubi JC, Krenning EP, Lamberts SW,Croughs RJ. Clinically nonfunctioning pituitary adenoma and octreotide response to long term high dose treatment, and studies in vitro. J ClinEndocrinolMetab. 1992; 75: 1310–1317. [PMID: 1430093][Pubmed]
[4]Gasperi M, Petrini L, Pilosu R, Nardi M, Marcello A, Mastio F, Bartalena L, Martino E. Octreotide treatment does not affect the size of most non-functioning pituitary adenomas. J Endocrinol Invest. 1993; 16: 541–543. [PMID: 8227984 [Pubmed]
[5]Gittoes NJ, Bates AS, TseW, Bullivant B, Sheppard MC, Clayton RN, Stewart PM. Radiotherapy for non-function pituitary tumors. ClinEndocrinol 1998; 48: 331–337. [PMID: 9578824][Pubmed]
[6Woollons AC, Hunn MK, Rajapakse YR, Toomath R, Hamilton DA, Conaglen JV,Balakrishnan V. Non-functioning pituitary adenomas: indications for postoperative radiotherapy. ClinEndocrinol (Oxf).2000; 53: 713–717. [PMID: 11155093].[Pubmed]
[7]Greenman Y, Ouaknine G, Veshchev I, Reider-Groswasser II, Segev Y, Stem N. Postoperative surveillance of clinically nonfunctioning pituitary macroadenomas: markers of tumor quiescence and regrowth. ClinEndocrinol (Oxf).2003; 58: 763–769. [PMID: 12780754] [Pubmed]
[8].Gittoes NJ. Radiotherapy for non-functioning pituitary tumors– when and under what circumstances? Pituitary 2003; 6: 103–108. [PMID: 14703020] [Pubmed]
[9]Bates AS, Van’t Hoff W, Jones PJ, Clayton RN. The effect of hypopituitarism on life expectancy. J ClinEndocrinolMetab.1996; 81: 1169–1172. [PMID: 8772595] [Pubmed]
[10].Littley MD, ShaletSM, Beardwell CG, Ahmed S R, Applegate G, Sutton ML. Hypopituitarism following external radiotherapy for pituitary tumors in adults. Q J Med. 1989 70 145–160. [PMID: 2594955 [Pubmed]
[11]Brada M, Ford D, Ashley S, Bliss JM, Crowley S, Mason M, Rajan B,Traish D. Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma. BMJ. 1992 May 23; 304(6838):1343-6. [PMID: 1611331].[Pubmed]
[12]Tsang RW, Laperriere NJ, Simpson WJ, Brierley J, Panzarella T, Smyth HS. Glioma arising after radiation therapy for pituitary adenoma. A report of four patients and estimation of risk. Cancer. 1993 Oct 1;72(7):2227-33. [PMID: 8374881 [Pubmed]
[13]McCord MW, Buatti JM, Fennell EM, Mendenhall WM, Marcus RB Jr, Rhoton AL, Grant MB, Friedman WA. Radiotherapy for pituitary adenoma: long-term outcome and squeal. Int J RadiatOncolBiol Phys. 1997 Sep 1; 39(2):437-44. [PMID: 9308948 [Pubmed]
[14]Losa M, Valle M, Mortini P, Franzin A, da Passano CF, Cenzato M, Bianchi S, Picozzi P,Giovanelli M. Gamma knife surgery for treatment of residual nonfunctioning pituitary adenomas after surgical debulking. J Neurosurg. 2004 Mar;100(3):438-44. [PMID: 15035279] [Pubmed]
[15]Lillehei KO, Kirschman DL, Kleinschmidt-DeMasters BK &Ridgway EC. Reassessment of the role of radiation therapy in the treatment of endocrine-inactive pituitary macroadenomas. Neurosurgery. 1998 Sep;43(3):432-8; discussion 438-9. [PMID: 9733298].[Pubmed]
[16]Bulow B, Hagmar L, Mikoczy Z, Nordstrom CH,Erfurth EM. Increased cerebrovascular mortality in patients with hypopituitarism. ClinEndocrinol (Oxf). 1997 Jan;46(1):75-81. [PMID: 9059561 [Pubmed]
[17]Asano K, Kubo O, Tajika Y et al. The relationship between cell proliferation and secretory activity in pituitary adenomas.A review of 63 cases. No ToShinkei. 1996 Jun;48(6):543-9. [PMID: 8703557 [Pubmed]
[18]MastronardiL, GuiducciA, SperaC, PuzzilliF, LiberatiF. Ki-67 labelling index and invasiveness among anterior pituitary adenomas: analysis of 103 cases using the MIB-1 monoclonal antibody. J ClinPathol. Feb 1999; 52(2): 107–111. [PMID: 10396237 [Pubmed]
[19]Buchfelder M, Fahlbusch R, Adams EF, Kiesewetter F, Thierauf P. Proliferation parameters for pituitary adenomas. ActaNeurochir Suppl. 1996;65:18-21. [PMID: 8738487 [Pubmed]
[20]Daita G, Yonemasu Y. Dural invasion and proliferative potential of pituitary adenomas. Neurol Med Chir (Tokyo). 1996 Apr;36(4):211-4. [PMID: 8741248 [Pubmed]
[21]Shibuya M, Saito F, Miwa T, Davis RL, Wilson CB, Hoshino T. Histochemical study of pituitary adenomas with Ki-67 and anti-DNA polymerase alpha monoclonal antibodies, bromodeoxyuridine labeling, and nucleolar organizer region counts. ActaNeuropathol. 1992; 84(2):178-83. [PMID: 1381860 [Pubmed]
[22]]. Ekramullah SM, Saitoh Y, Arita N,Ohnishi T, Hayakawa T. The correlation of Ki-67 staining indices with tumor doubling times in regrowing non-functioning pituitary adenomas. ActaNeurochir (Wien) 1996;138:1449–55. [PMID: 9030353 [Pubmed]
[23]Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PJ, Murray D, Laws ER Jr. Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB-1 antibody. Neurosurgery. 1996 Jan;38(1):99-106; discussion 106-7. [PMID: 8747957 [Pubmed]
[24]Landolt AM, Shibata T, Kleihues P. Growth rate of human pituitary adenomas. J Neurosurg 1987;67:803–6 [PMID: 3681419 [pubmed]
[25]Knosp E, Kitz K, Perneczky A. Proliferation activity in pituitary adenomas: measurement by monoclonal antibody Ki-67. Neurosurgery. 1989 Dec;25(6):927-30. [PMID: 2601824][Pubmed]
[26]Abe T, Sanno N, Osamura YR, Matsumoto K. Proliferative potential in pituitary adenomas; measurement by monoclonal antibody MIB-1.ActaNeurochir (Wein) 1997; 139:613-8. [PMID: 9265953] [Pubmed]
[27]Yonezawa K, Tawaki N, Kokunai T. Clinical features and growth factors of pituitary adenomas. Surg Neurol. 1997 Nov;48(5):494-500. [PMID: 9352815 [Pubmed]
[28]Hentschel SJ, Mc Cutcheon E, Moore W, Durity FA. P53 and MIB-1 immunohistochemistry as predictor of the clinical behavior of nonfunctioning pituitary adenomas. Can J of NeurolSci; 2003: 30: 215-9. [PMID: 12945944 [Pubmed]
[29]Wolfsberger S. expression of cell proliferation markers in pituitary adenomas-corellation and clinical relevance of MIB-1 and anti-topoisomerase-II alpha. ActaNeurochir (Wein) 2004; 146: 831-9. [PMID: 15254805].[Pubmed]
[30]Rishi A, Sharma MC, Sarkar C, Jain D, Singh M, Mahapatra AK, Mehta VS, Das TK. A clinicopathological and immunohistochemical study of clinically nonfunctioning pituitary adenomas: A single institutional experience. Neurol India 2010; 58:418-23. [PMID: 20644271] [Pubmed]

