Research

Mesogenic Complexes of Some Transition Metal Ions of the Mesogenic Schiff-Base, N,N’-1 di-(4’-heptyloxybenzoatesalicylidene)-l’’, 8’’-diamino-3’’, 6’’-dioxaoctane: Synthesis and 2 Spectral Studies 3
*Sanyucta Kumari
- Submitted: Monday,Monday, March 17, 2014
- Accepted: ;Monday, March 24, 2014
- Published: Monday, March 24, 2014
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ((http://creativecommons.org/licenses/by/3.0)which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
A mesogenic Schiff-base, N,N’-di-(4’-heptyloxybenzoatesalicylidene)-l’’, 8’’-diamino- 3’’,6’’-dioxaoctane; H2dobsdd (H2L1), that nematogenic mesophase was synthesized and its structure studied by elemental analysis and FAB mass, NMR& IR spectra. The Schiff-base, H2L1, upon condensation with hydrated lanthanide(III) nitrates, yields LnIII complexes of the general composition [Tm2(L1H2)3(NO3)4](NO3)2, whereTm =Sc,Ti,Vd,Cr. The IR and NMR spectral data imply a bi-dentate of the Schiff-14 base through two phenolate oxygens in its zwitterionic form (as L1H2) to the LnIII ions, rendering the overall geometry of the complexes to seven-coordinated polyhedron - possibly distorted mono-capped octahedron. Among the metal complexes, only that of Ti and Vd are found to be mesogenic.
Keywords
Schiff-base, Mesogenic, Zwiterionic Coordination, Mono-capped octahedron, NMR 20 & IR spectra.
Introduction
Metallomesogens [1,2] were considered promising materials with novel and interesting properties, based on the role of metal ions in the formation of molecular motifs and intermolecular interactions, which were unapproachable for organic liquid crystals [3,5]. As a part of our detailed research investigation [6,10] on transition metal complexes of a series of mesogenic Schiff' base ligands derived from 2, 4- dihydroxybenzaldehyde, we report here the synthesis and spectral studies of some 3d metal complexes of N,N’-di-(4’-30 hepttyloxybenzoatesalicylidene)-l’’, 8’’-diamino-3’’,6’’-dioxaoctane; H2dobsdd, HL1. We have observed that the TiII complex of HL1, I (scheme. 1), was reported [11] and the structure discussed on the basis of powder XRD, mesogenic and magnetic moment data; however, the bonding nature of carbonyl oxygen was not reported due to lack of IR and NMR spectral studies. Further, Hoshino et al. [12] reported a similar CuII complex, II, where the two terminal alkyl chains of I were interchanged, and established the bonding nature of carbonyl oxygen on the basis of single crystal XRD data with an octahedral environment around the metal ion. In the present work we report a square-planar Sc II and octahedral TiII, VdII, and CrII complexes on the basis of a logical discussion of spectral data on the bonding nature of ligand (HL1).
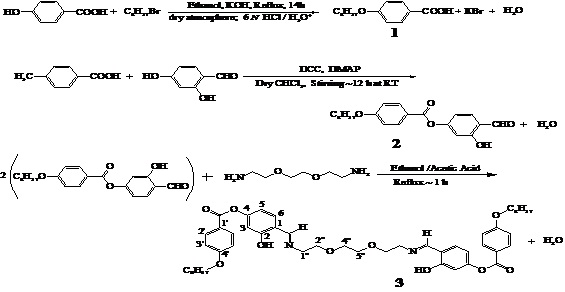
Scheme-1: Synthetic steps involved in the synthesis of 1 (p-octyloxybenzoic acid), 2 (4-octyloxy-(4’-formyl-3’-hydroxy)benzoate) and 3N,N’-di-(4’-octyloxybenzoate salicylidene)-l’’,8’’-diamino-3’’,6’’-dioxaoctane (H2L3)
Experimental Section
Materials
All the required reagents of analytical grade (AR) were purchased from commercial sources and used without further purification: 1-bromooctane, 2, 4-dihydroxybenzaldehyde, N,N’-dicyclohexylcarbodiimide (DCC), N,N-di methylaminopyridine (DMAP) and 1,8-diamino-3,6-dioxaoctane are from Sigma-Aldrich, USA; all Ln(NO)3.xH2O are from Indian Rare Earths Ltd. and KOH from Merck. The organic solvents purchased obtained from commercial vendors were dried using standard methods [15] when required.
Synthesis and analysis
N,N’-di-(4’-octyloxybenzoatesalicylidene)-l’’,8’’-diamino-3’’,6’’-dioxaoctane (H2L3), 3 (scheme. 1), was synthesized from the precursor materials, 1 and 2, as earlier reported method [9,12,13].[Ln2(LiH2)3(NO3)4](NO3)2: (Tm =Sc, Ti,Vd andCr ) complexes were prepared by refluxing together solutions of the ligand, H2L1 (2.56g, 3.0 mmol in 30 mL dichloromethane) and the appropriate metal nitrate (1.0 mmol, in 20 mL ethanol) for ~ 2h at 40 0C. The reaction mixture was left over night in the flask closed with guard tube. The solid complex that separated out in each case was filtered under suction and washed repeatedly with cold ethanol and dried over fused CaCl2.
Synthesis of N,N’-di-(4’-heptyloxybenzoatesalicylidene)-l’’,8’’-diamino-3’’,6’’- dioxaoctane; H2dobsdd H2L1,3 :
The ligand, N,N’-di-(4’-heptyloxybenzoatesalicylidene)-l’’,8’’-diamino-3’’,6’’-dioxa octane (H2L1) was prepared by refluxing together absolute ethanolic solutions of 4-octyloxy-(4’- formyl-3’-hydroxy)benzoate (14.80 g, 40 mmol, in 100 mL) and l,8-diamino-3,6-dioxaoctane (2.95 mL, 25 mmol in 10 mL) for ~1 h in presence of a few drops of acetic acid left over night in 66 the flask closed with guard tube. The micro-crystalline product, 3, was suction-filtered, thoroughly washed with cold ethanol and recrystallized from solution of absolute ethanol / Chloroform (v/v, 1/1) and dried at room temperature. Yield: 11.10 g (65%) as a yellow coloured solid; m.p. 100 0C. 1H NMR: (300.40 MHz; CDCl3; Me4Si at 25 °C, J (Hz), ppm) δ= 0.90(t, 3JH-H 70 = 6.0, 3H, CH3), 1.82-1.31(m, 12H, CH2), 3.62(s, 2H, H4’’), 3.73(s, 3JH-H = 6.6 Hz, 2H, H1’’/ 71 H2’’), 4.04(t, 3JH-H = 6.6, 2H, H1’’’), 6.72(dd, 3JH-H = 8.1, 4JH-H = 2.7, 1H, H5 ), 6.80(d, 4JH-H = 72 2.7, 1H, H3), 6.96(dd, 3JH-H = 6.6, 4JH-H = 2.4, 1H, H3’), 7.27(dd, 3JH-H = 8.7, 4JH-H = 3.0, 1H, H6), 73 8.11(dd, 3JH-H = 6.0, 4JH-H = 3.0, 1H, H2’), 8.33(s, 1H, N=CH), 13.82(br s, 1H, Ph-OH); 13C{1H} 74 NMR: (75.45 MHz; CDCl3; Me4Si at 25 °C, ppm) δ=165.72 (-COO), 164.46(-C4’), 163.62(-C2), 75 163.28(-NCH), 154.27(-C4), 132.34(-C6), 132.23(-C2’), 121.34(-C1’), 116.63(-C1), 114.32(-C5), 76 112.32(-C3’), 110.59(-C3), 70.67(-C2’’), 70.42(-C4’’), 68.35(-C1’’), 58.57(-C1’’’), FAB Mass: The 77 molecular ion (m/e, 853; 50% intensity) generates simultaneously two fragments, M1 and M1’, 78 (m/e, fragment, % intensity): M1: 233, C8H17OC6H4CºO+, 100; M1’ : 121, HOC6H4CºO+, 70%; 79 IR (KBr, cm-1): 3443br n(O-H)phenol, 1724vs n(>C=O), 1633s n(C=N), 1168s n(C-O)phenolic; 80 C50H64N2O10 (853.05): Calcd. C 70.40, H 7.56, N 3.28; found C 70.42, H 7.59, N 3.24.
Synthesis of the TmII Complex, [Sc2(L1H2)3(NO3)4](NO3)2:
Anhydrous solutions of N,N’-di-(4’-heptyloxybenzoatesalicylidene)-l’’,8’’-diamino- 3’’,6’’-dioxaoctane (2.56g, 3 mmol in 30 mL dichloromethane) and of Tm(NO3)3.6H2O (0.43, 1 mmol in 20 mL ethanol) were refluxed for ~ 2h and the reaction mixture left over-night in the reaction flask. The crude solid complex, was filtered off under suction, washed repeatedly with ethanol, recrystallised from the solution of chloroform/ethanol and dried over fused CaCl2. Yield: 1.89 g (59%); as a yellow coloured solid; m.p. 210 0C (decompose); 1H NMR: (300.40 89 MHz; DMSO-d6; Me4Si at 25 °C, J (Hz), ppm) δ= 0.87(t, 3JH-H = 5.1, 3H, CH3), 1.77-1.27(m, 90 12H, CH2), 3.56(s, 2H, H4’’), 3.68(s, 3JH-H = 6.6 Hz, 2H, H1’’/ H2’’), 4.07(t, 3JH-H = 6.0, 2H, H1’’’), 7.08(d, 3JH-H = 8.4, 1H, H5 ), 6.72(s, 1H, H3), 7.47(d, 3JH-H = 8.7, 1H, H6), 8.03(d, 3JH-H = 8.4, 92 1H, H2’), 8.53(s, 1H, N=CH), 12.08(br-s, 1H, -N+H); 13C{1H} NMR: (75.45 MHz; DMSO-d6; Me4Si at 25 °C, ppm) δ=166.27(-COO), 163.97(-C4’), 163.78(-C2), 189.28(-NCH), 154.12(-C4), 132.85(-C6), 132.07(-C2’), 121.61(-C1’), 116.14(-C1), 114.75(-C5), 111.90(-C3’), 110.54(-C3), 69.78(-C2’’), 69.70(-C4’’), 68.02(-C1’’), 56.87(-C1’’’), IR (KBr, cm-1): n(O-H)phenol absent, 1730vs 96 n(>C=O), 1657s n(C=N), 1151s n(C-O)phenolic; La2C150H192N12O48(3208.99): Calcd. C 56.14, H 97 6.03, N 5.24, La 8.66; found C 56.10, H 5.27, N 5.26, La 8.62.All the other rare-earth complexes (Ln = Pr, Nd, Sm, Eu, Gd, Tb, Dy and Ho) were synthesized in an analogous way by using the appropriate hydrated salt of LnIII nitrate. The Physical property and infrared spectral data of two representative complexes are given below:
Sc Complex, [SC2(L1H2)3(NO3)4](NO3)2:
Yield: 1.91 g (59%) as a yellow coloured solid; m.p. 0C(decompose); IR (KBr, cm-1): n(O-H)phenol absent, 1732vs n(>C=O), 1657s n(C=N), 1151s n(C-O)phenolic; Gd2C150H192N12O48(3245.68): Calcd. C 55.51, H 5.96, N 5.18, Gd 9.69; found C 55.48, H 5.99, 106 N 5.20, Gd 9.67.
Ti Complex, [Ti2(L1H2)3(NO3)4](NO3)2:
Yield: 2.12 g (65%); as a yellow coloured solid m.p. 190 0C(decompose); IR (KBr, cm-1): n(O-H)phenol absent, 1734vs n(>C=O), 1655s n(C=N), 1151s n(C-O)phenolic; Ho2C150H192N12O48(3261.04): Calcd. C 55.25, H 5.93, N 5.15, Ho 10.12; found C 55.28, H 6.00, N 5.19, Ho 10.08.
Physical measurements
The 1H and 13C NMR spectra were recorded on a JEOL AL-300 MHz FT-NMR multinuclear spectrometer; C, H, and N contents were micro analyzed on an Elemental Vario EL III Carlo Erba 1108 analyzer. Infrared spectra were recorded on a JASCO FT/IR (model-5300) spectrophotometer in the 4000-400 cm-1 region. The mass spectra were recorded on JEOL SX- FAB mass spectrometers. The UV-VIS spectra were recorded on a Shimadzu spectrophotometer, model, Pharmaspec, UV 1700. Magnetic susceptibility measurements were made at room temperature on a Cahn-Faraday balance balance using Hg[Co(NCS)4] as the calibrant. Mesophases were identified by the textures observed by using polarized hot-stage binocular microscope (LOMO, USA) equipped with digital camera (Nikon Coolpix 4500). Differential Scanning Calorimetry studies were made on METTLER DSC-25, Mettler STARe SW 9.00 unit.
Results and Discussion
Spectral, Magnetic and thermal investigation
The yellow- colored mesogenic Schiff-base ligand (H2L1) was react with Tm(III) nitrates to yield yellow/cream colored LnIII complexes. The parent ligand as well as the metal complexes are soluble in DMF and DMSO (expect ligand soluble in chloroform and dichloromethane) but are insoluble in water, methanol, ethanol and other common organic solvents. In all the metal complexes under discussion, it is the non-deprotonated species of the ligand that has been found to coordinate to the metal ion and the nitrate ions counter balance the positive charge of the LnIII ion(s); the nitrate groups were found to be present both within the coordination sphere as well as outside the sphere; the number of the ionic species was implied by the molar conductance data (154-160 ohm-1 cm2/mole) measured in 10-3 M solutions of DMF of the complexes which imply 2:1 electrolytic behaviour [16].
The structures and purity of the Schiff-base ligand and of the metal complexes were studied by IR and NMR spectroscopy and elemental analyses. The structure of the ligand was further confirmed by FAB Mass spectrum. The mass spectral features of H2L3 were characterized by the molecular ion peak corresponding to the m/e value of 853, which matches with the molecular weight of 853.05 of H2L3 of the molecular formula, C50H64N2O10. The 100% intensity of the base peak is as expected for the fragment (C8H17O-(C6H4)-CºO+, m/e = 233) on the basis of its predominant aromatic character; the other major fragment peak (m/e = 121) is due to HO(C6H4)-CºO+.
A comparison of the 1H and 13C{1H} NMR spectral data of the ligand with that of the LaIII complex shows the absence of the phenolic-OH signal. From the composition of the LaIII complex, [La2(L3H2)3(NO3)4](NO3)2, phenolic-OH signal, appearing at d, 13.82 in the former, disappears upon complexation due to phenolic protons are shifted to the two uncoordinated imino nitrogens, which then get intramolecularly hydrogen-bonded to the metal-bound phenolate oxygens to give rise to the zwitter-ionic structure (=N+–H• • •O–). The macrocycle under this condition is designated as L3H2 [17]. We found that the signal corresponding to the imine hydrogen, -CH-N, was in the Tm complex (d, 8.53) when compared with that of the Schiff base ligand (d, 8.33); further, a new signal, characteristic of –N+H resonance, appears in the spectrum of the LaIII complex at 14.03 d while the parent ligand does not show any such signal. Binnemans et al. [18], while reporting their work on rare earth-containing magnetic liquid crystals of the formula, [Tm(LH)3(NO3)3], where LH = 4-alkoxy-N-alkyl-2-hydroxy benzaldimine, found that selective irradiation of the signal at 12.29d removed the broadening of the imine signal, thereby inferring that the signal does not correspond to the proton of the -OH group, but to the proton of the -N+H group. These observations confirm that the Schiff base is present in the metal complex in a zwitterionic form, with the phenolic oxygen deprotonated and the imine nitrogen protonated (scheme. 2).

Scheme 2: Depiction of migration of phenolic protons to imine nitrogens of the ligand, H2L1, during the formation of zwitter ion
The broad absorption centered on 3443 cm-1 in the IR spectrum of the parent ligand, characteristic of n(O-H)phenolic [19] may be understood to involve considerable amount of H- bonding (presumably intramolecularly bonded to the ortho >C=N group) under the experimental conditions; this band totally disappears in the spectra of LnIII the complexes due to the shifting of the phenolic proton to the azomethine nitrogen atom resulting in the formation of zwitter ion. The weak/medium intensity bands centered on 1151 cm-1 are assignable to n(C-O)phenolic, the strong intensity band occurring at 1633 cm-1, assignable [20] to n(C=N) absorption of the azomethine moiety, undergoes a hypsochromic shift in all the complexes on account of zwitter- ion formation. Thus, the process of complexation of the ligand with LnIII ions resulted in shifting of the phenolic protons to the two uncoordinated imino nitrogens, which then get intramolecularly hydrogen-bonded to the metal-bound phenolate oxygens to give rise to the zwitterionic structure, =N+–H• • •O−. Similar zwitter-ionic behaviour has been reported by others for acyclic Schiff base lanthanide complexes [21]. The formation of a zwitter-ionic form can be rationalized by the tendency of the lanthanide ions to coordinate to negatively charged ligands with a preference for O-donor ligands. By transfer of the phenolic proton to the imine nitrogen, the phenolic oxygen becomes negatively charged and can coordinate to the lanthanide ion. The initial evidence for zwitter-ionic formation was obtained from 1H NMR spectra while further evidence was given by infrared spectroscopy and, more particularly, by the band frequencies of the n(C=N) vibration. The shift to higher wave numbers in the complexes compared to the corresponding values in the ligands indicates that the nitrogen atom is notinvolved in the complex formation and that a C-N+ group is present [22]. Further, all the complexes are characterized by a strong band due to n(C=N) at 1657(24) cm−1 and a weak broad band at about 3049 cm−1 due to the hydrogen bonded N+–H • • •O- vibration of the protonated imine [22]. Thus, the ligands coordinate to the metal ion via the negatively charged phenolic oxygen only; no binding occurs between the lanthanide ion and the imine nitrogen. The LnIII complexes also exhibit three additional bands around 1488–1473, 1258–1255, and 847-844 cm-1, which can be assigned to the vibrational modes of the coordinated (C2v) nitrate groups [23]. The magnitude of splitting at higher energies, 231–215 cm-1, suggests that the coordinated nitrate groups act as bidentate ligands [23,24]. The additional bands observed at 1385–1383 cm-1 are due to the non-coordinated nitrate present in the complexes. At this juncture, it may be recalled that M:L (2:3) ratios of the complexes with in this series, viz., [Tm2(L1H2)3(NO3)4](NO3)2 and where TmII = Sc, Ti, Vd and Cr with two different modes of nitrate group (ionic as well as bidentate-coordinate) in both types. At this juncture, a distorted mono-capped octahedron with C.N. = 7 may be tentatively proposed for the complexes (Fig 1) .
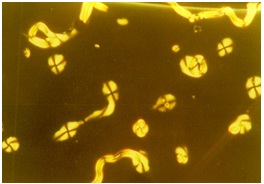
Fig 1 Optical Texture of Ti Complex
The meff values (at R.T.) of the present LnIII complexes (2.11, 6.86, 1.27, 1.88, 4.84, 7.63, 8.91 and 7.92 B.M. respectively where Ln = Pr, Nd, Sm, Eu, Gd, Tb, Dy, and Ho respectively), those have been found to be slightly lower / lower than the reported Van vleck values which may be on account of weak metal-metal interactions (antiferromagnetism) [25,27].
The electronic spectra (qualitative spectra in solution state in a mixed solvent of CHCl3 and DMSO (3:1 v/v) in the 200-1100 nm region) of only the PrIII, NdIII, SmIII and DyIII complexes were recorded. All the present complexes show considerable red shifts in the λmax values as compared to the aqua ion [28] presumably due to Nephelauxetic effect [29]. Various bonding parameters (Table 1) viz., Nephe1auxetic ratio (β), bonding parameter (b1/2), Sinha's parameter (%δ) and covalency angular overlap parameter (η), calculated by the procedures as reported in literature [30], suggest weak covalent nature of the metal-ligand bonds.
|
Sc
|
Vd
|
Ti
|
Cr
|
|
Transitions / Bonding parameters
|
lmax
(cm-1)
aq. Ion
|
lmax
(cm-1)
Complex
|
Transitions / Bonding parameters
|
lmax
(cm-1)
aq. Ion
|
lmax
(cm-1)
Complex
|
Transitions / Bonding parameters
|
lmax
(cm-1)
aq. Ion
|
lmax
(cm-1)
Complex
|
Transitions / Bonding parameters
|
lmax
(cm-1)
aq. Ion
|
lmax
(cm-1)
Complex
|
|
1G4!3H4
|
9900
|
9834
|
4F3/2!4I
9/2
|
11450
|
11198
|
6F9/2!6H5/2
|
9200
|
9157
|
6H5/2!6H15/2
|
10200
|
9980
|
|
1D2*
|
16850
|
16502
|
4F5/2,
2H9/2
|
12500
|
12484
|
6F11/2
|
10500
|
10471
|
6F5/2
|
12400
|
12019
|
|
3P0
|
20800
|
19342
|
4S3/2,
4F7/2
|
13500
|
13422
|
4G5/2
|
17900
|
17857
|
6F3/2
|
13200
|
13123
|
|
|
|
|
4F9/2
|
14800
|
14598
|
4M15/2
|
20800
|
20533
|
4F7/2
|
25800
|
25773
|
|
|
|
|
2G7/2*
|
17400
|
17391
|
6P7/2*
|
26750
|
26315
|
4K17/2
|
26400
|
-
|
|
|
|
|
4G9/2
|
19500
|
-
|
|
|
|
|
|
|
|
|
|
|
2P3/2
|
26300
|
26525
|
|
|
|
|
|
|
|
β
|
|
0.961
|
|
|
0.998
|
|
|
0.990
|
|
|
0.989
|
|
b1/2
|
|
0.139
|
|
|
0.032
|
|
|
0.071
|
|
|
0.074
|
|
%
δ
|
|
4.058
|
|
|
0.200
|
|
|
1.010
|
|
|
1.112
|
|
η
|
|
0.020
|
|
|
0.001
|
|
|
0.005
|
|
|
0.006
|
*Hypersensitive band
The mesophases were identified by polarized optical microscopy (POM) and DSC techniques. The POM study of mesogenic Schiff-base, displaying nematic (N) phase (Table 2). Typical for the nematic phase is the Schlieren texture with two and four brushes, the nematic phase separates from the isotropic liquid as droplets. Among the all LnIII complexes of H, only LaIII and GdIII complexes were found to be mesogenic where the former showed nematic mesophases while the latter displayed smectic-A (SmA) mesophase (Fig 2).
|
Compounds
|
Transition Temperature (°C)
|
|
H2L3
|
Crys · 87.02 ·
N · 99.39 · I
I · 95.32 · N · 80.51 ·Crys
|
|
[Ti(L3H2)3(NO3)4](NO3)2
|
|
|
[Vd2(L3H2)3(NO3)4](NO3)2
|
Crys · 169.37 ·
SmA · 210dec
|
Abbreviations: Crys = Crystalline phase, SmA = Smectic A phase, N =
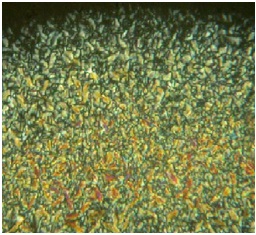
Fig 2 Optical Texture of Vd Complex
Conclusions
The nematogenic Schiff-base, N,N’-di-(4’-heptyloxybenzoatesalicylidene)-l’’,8’’ diamino-3’’,6’’-dioxaoctane, (H2L1) coordinates to LnIII as a neutral bidentate species, to yield yield seven-coordinate complexes (the polyhedron being possibly distorted mono-capped octahedron) of the general formula, [(Tm1H2)3(NO3)4](NO3)2, where Tm = Sc,Ti, Vd and Cr. Among all the complexes, only the Ti and Vd complexes exhibit liquid crystalline property. (nematic and smectic-A phases). In all cases, the zwitterionic-species of the neutral bi-dentate ligand, H2L1, coordinates to the LnIII metal ion through two phenolate oxygens.
Acknowledgements
The authors wish to acknowledge the recording of FAB Mass spectra and elemental analyses at the Central Drug Research Institute, Lucknow. One of the authors, Sanyucta Kumari, gratefully acknowledges the financial grant received from the Council of Scientific & Industrial Research, New Delhi [vide grant No. 9/13(233)/2009-EMR-I dated 08-05-2009].
Conflict of Interests
None
Authors’ Contribution
The author drafted and edited the manuscript
Funding
None
Ethical Considerations
None
References
[1]Spinu, C., and Kriza, A.(2000) Acta. Chim. Slov., 47:179-185.
[2]Sun, B., Chen, J., Hu, J.Y., and Li, X. (2001) J. Chin. Chem. Lett., 12:1043-1050.
[3].Boghaei, D.M., and Mohebi, S. (2002) Tetrahedron, 58:5357-5366.
[4]Liu, J., Wu, B., Zhang, B., and Liu, Y. (2006) Turk. J. Chem., 30:41-48.
[5]P.A. Vigato, S. Tamburin, Coord. Chem. Rev. 248 (2004) 717.
[6]W. Radecka-Paryzek, V. Patroniak, J. Lisowski, Coord. Chem. Rev. 249 (2005) 2156.
[7]M. Tsiouria, N. Hadjiliadis, T. Arslan, B.M. Kariuki, J.C. Plakatouras, Inorg. Chem. Commun. 9 (2006) 429.
[8]] K. Binnemans, K. Lodewyckx, Angew. Chem. 113 (2001) 248. [pubmed]
[9]Angad Kumar Singh, Sanyucta Kumari, Pawan Raj Shakya and T. R. Rao, Materials Science and Engineering: C, 31(2011) 1111-1114.
[10]Sanyucta Kumari, Angad Kumar Singh and T. R. Rao, Materials Science and Engineering: C, 29 (2009) 2454-2458.
[11]Sanyucta Kumari, Angad Kumar Singh, K. Ravi Kumar, B. Sridhar and T. R. Rao, Inorg. Chim. Acta 362 (2009) 4205–4211.
[12]Angad Kumar Singh, Sanyucta Kumari, T. N. Guru Row, Jai Prakash, K. Ravi Kumar, B. Sridhar and T. R. Rao, Polyhedron 27 (2008) 3710-3716.
[13]] Angad Kumar Singh, Sanyucta Kumari, K. Ravi Kumar, B. Sridhar and T. R. Rao, Polyhedron 27 (2008) 181-186.
[14]N.V.S. Rao, M. K. Paul, T. R. Rao and A. Prasad, Liquid Crystals 29 (2002) 253 1243.
[15]A. Weisberger and F.S.Praskver, Organic solvents, International publishers Inc., 255 New York, 1956 p. 1263.
[16]W. J. Geary, Coord. Chem. Rev. 7 (1971) 81-122.
[17]P. Bag, U. Flörke and K. Nag, Dalton Trans (2006) 3236-3248.
[18]K. Binnemans, Y. G. Galyametdinov, R. Van Deun, D. W. Bruce, S. R. Collison, A. P. Polishchuk, I. Bikechantaev, W. Hasse, A. V. Prosvirin, L. Tinchurina, I. Litvinov, A. Gubajdullin, A. Rakhamatullin, K. Uytterhoeven and L. 261 VanMeervelt, J. Am. Chem. Soc. 122 (2000) 4335-4344. 262
[19]R. M. Silverstein and F. X. Webster, Spectrometric Identification of organic Compounds, John-Wiley& Sons, Inc. New York, 6th ed., 2002 p.82, 83, 87-88, 90-91.
[20] N. B. Colthup, L. H. Daly, and S. E. Wiberley, Introduction to Infrared and Raman Spectroscopy,3rd Ed., Academic Press, New York 1990.
[21]K. Binnemans, D. W. Bruce, S. R. Collison, R. Van Deun, R. Galyametdinov and Y. G. Martin, Philos. Trans. R. Soc. London, Ser. A 357 (1999) 3063-3077.
[22]B. Keshavana, P. G. Chandrashekaraa and N. M. Made Gowdab, Journal of Molecular Structure 553 (2000) 193-197.
[23]K. Binnemans, R. Van Deun, Christiane G. Walrand, W. Haase, D. W. Bruce, L. Malykhina, and Y. G. Galyametdinov, Materials Science and Engineering C 18 (2001) 247-254.
[24]J. Nawrocka and V. Patroniak, Journal of Alloys and Compounds 380 (2004) 159-275 162.
[25].W. Plass and G. Fries, Z. Anorg. Allg. Chem. 623 (1997) 1205-1207.
[26]J. P. Costes, F. Dahan, A. Dupuis, S. Lagrave and J. P. Laurent, Inorg. Chem. 37 (1998) 153-155.[pubmed]
[27]J. P. Costes, A. Dupuis and J. P. Laurent, Inorg. Chim. Acta 268 (1998) 125-130.
[28]R. Reisfe1d and C.K. Jorgensen, Lasers and Excited States of Rare Earths, 281 Springer-Verlag, Berlin, 1977.
[29]W. T. Carnall, P. R. Fields and K. Rajnak, J. Chern. Phys. 49 (1968) 4424-4442.
[30]T. R. Rao and P. A. Kumar, Synth. Rect. Inorg. Met.-Org.Chem. 25 284 (1995) 1011-1026.

