Research

Second malignancy of the oral cavity after brachytherapy for tongue cancer in long-term follow-up patients
1,2Eri Omoto, 2Hitoshi Shibuya2, 2Keiko Nakagawa,2,Kiyomi Amemiya,2Keiji Hayashi
- 1Department of Radiology, NTT Medical Center Tokyo, Tokyo, 141-8625, Japan
- 2Department of Diagnostic Radiology and Oncology, Tokyo Medical and Dental University, Tokyo, 113-8519, Japan
- Submitted:Friday, January 24, 2014
- Accepted:Saturday, February 08, 2014
- Published:Wednesday, February 19, 2014
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Introduction
Brachytherapy is as effective as surgery for tumor control, with better functional and cosmetic results. When the primary treatment includes radiation therapy, however, a second malignancy (SM) may develop in the previously irradiated area. The present study investigated the incidence and treatment results of SM in the oral cavity after brachytherapy for tongue cancer.
Study design
A retrospective study was performed using medical records from our institution’s database.
Methods and Materials
A retrospective review of 281 patients who had been treated with brachytherapy for stage I or II tongue cancer between 1965 and 2000 was performed. All of the patients had been followed-up for more than 10 years at the Department of Diagnostic Radiology and Oncology, Tokyo Medical and Dental University Hospital.
Results
SM of the oral cavity occurred in 26 (9.3%) of the 281 patients between 8 and 32 years after brachytherapy. Twenty of these patients underwent surgery, and their 4-year overall survival rate was 94.4%. Four patients received a second brachytherapy treatment because of the presence of inoperable and/or unresectable lesions, but all of these treatments failed; 3 of the patients died of SM at a median of 1.8 years after the diagnosis. One patient received laser treatment and another patient received chemotherapy, but both of these patients died of uncontrolled SM at 2 months and 5 months after the diagnosis of SM.
Conclusions
SM can occur in the oral cavity after brachytherapy for tongue cancer during a long-term follow-up period. In such cases, surgery is the only successful salvageable treatment.
Keywords
tongue cancer, treatment results, brachytherapy.
Introduction
As diagnostic and therapeutic techniques for head and neck squamous cell carcinoma continue to improve, the expectation is that the relative survival rates will likely increase.
Brachytherapy has been performed as one of the standard treatments for early-stage tongue cancer. Because brachytherapy delivers a high radiation dose to a limited volume while sparing the surrounding normal tissues, brachytherapy is as effective as surgery for tumor control, with better functional and cosmetic results [1,2].
Ionizing radiation, including X rays and gamma rays, has long been established as being carcinogenic, in addition to tobacco or alcohol exposure [3], and second malignancy (SM) has a reported incidence of 5%-30% following the successful treatment of head and neck tumor and is considered an important negative prognostic maker [4]. However, to our knowledge, SM after brachytherapy for tongue cancer has not been previously reported.
When a primary treatment includes radiation therapy, SM can develop in a previously irradiated area. The treatment of preference in cases of SM in a previously irradiated area is salvage surgery. Although repeat brachytherapy has been reported as being effective for the residual or recurrent cancer in a previously irradiated area with short-term follow-up in our institution [5], there has been no data regarding repeat brachytherapy for SM in the oral cavity with long-term follow-up.
We retrospectively examined the incidence of SM in the oral cavity in the patients who were followed- up for more than 10 years after brachytherapy for tongue cancer and analyzed the treatment results.
Material and methods
Between 1965 and 2000, 281 patients with stage I or II tongue cancer were treated with interstitial brachytherapy using low-dose-rate sources and, thereafter, were followed-up for more than 10 years at the Department of Diagnostic Radiology and Oncology, Tokyo Medical and Dental University Hospital. The patients consisted of 114 females and 167 males, and their age ranged from 19 to 82 years, with a median age of 55 years at the time of brachytherapy. According to the UICC TNM Classification of Malignant Tumors seventh edition, 89 patients had stage I (T1N0) disease and 192 patients had stage II (T2N0) disease. The histological diagnosis of all the tongue cancers was squamous cell carcinoma. The brachytherapy sources were Ra-226 needles in 135 patients, Ir-192 pins in 87 patients, Au-198 grains in 27 patients, Rn-222 seeds in 18 patients, Cs-137 needles in 9 patients, and Co-60 needles in 5 patients. The patients underwent brachytherapy alone and were irradiated with 70 Gy over about 7 days with a 5-mm margin around the tumor region [6,7]. The patients did not undergo chemotherapy. The follow-up period ranged from 10 to 51 years (median, 14.4 years).
We reviewed these tongue cancer patients and investigated the occurrence of SM in the oral cavity as well as the treatment outcome after obtaining the approval from the research ethics board committee. The actuarial curves for the incidence of SM, the overall survival rate, the local control rate, and the disease-free survival rate were calculated according to the Kaplan-Meier method. The statistical analyses were performed using SPSS version 19 software, and a univariate analysis was assessed using the log-rank test or chi-square test. A P-value less than 0.05 was considered significant.
Result
SM in the oral cavity was found in 26 (9.3%) of the 281 patients after brachytherapy for tongue cancer (Table 1). The interval between brachytherapy and the occurrence of SM ranged from 8 to 32 years, with a median of 11.7 years, and the 10-, 20-, and 30-year cumulative incidence rates were 2.5%, 10.3%, and 17.7%, respectively [Fig 1]. No recurrence or metastasis was found in any of the 26 patients at the time of the occurrence of SM. The patient age ranged from 51 to 87 years, with a median of 71 years at the time of the diagnosis of SM. The histological diagnosis was squamous cell carcinoma in 24 patients, granular cell carcinoma in 1 patient, and spindle cell carcinoma in 1 patient. SM occurred in the tongue in 21 patients, the lower gum in 2 patients, the buccal mucosa in 1 patient, the floor of the mouth in 1 patient, and the lip in 1 patient; all the SMs occurred on the side of the initial treatment. No significant differences between the incidence of SM and the characteristics of the initial tongue cancer (disease stage, patient age, sex, or brachytherapy source) were seen (Table 2).
Table 1: Summary of 26 patients with a second malignancy (SM)
|
Case number |
Stage |
Age at diagnosis (years) |
Sex |
Type of irradiation |
Latency in years and months |
Site of
the second malignancies |
Histopathological diagnosis of
the second malignancies |
Modality of treatment for the second malignancies |
| 1 |
T1 |
81 |
Male |
Ra-226 |
27y1m |
Tongue |
Squamous cell
carcinoma |
Au-198 |
| 2 |
T1 |
74 |
Female |
Rn-222 |
31y1m |
Tongue |
Squamous cell
carcinoma |
Surgery |
| 3 |
T1 |
63 |
Female |
Ra-226 |
32y0m |
lower gum |
Squamous cell
carcinoma |
Surgery |
| 4 |
T1 |
84 |
Male |
Ir-192 |
13y2m |
Tongue |
Squamous cell
carcinoma |
Au-198 |
| 5 |
T1 |
80 |
Male |
Ir-192 |
21y10m |
Tongue |
Squamous cell
carcinoma |
Surgery |
| 6 |
T1 |
51 |
Male |
Au-198 |
11y3m |
Tongue |
Squamous cell
carcinoma |
Surgery |
| 7 |
T1 |
79 |
Female |
Ir-192 |
8y0m |
Floor of mouth |
Squamous cell
carcinoma |
Surgery |
| 8 |
T1 |
71 |
Male |
Au-198 |
10y5m |
Tongue |
Squamous cell
carcinoma |
Surgery |
| 9 |
T1 |
64 |
Male |
Ra-226 |
13y4m |
Lip |
Granular cell
carcinoma |
Surgery |
| 10 |
T2 |
59 |
Female |
Rn-222 |
9y1m |
Tongue |
Squamous cell
carcinoma |
Rn-222 |
| 11 |
T2 |
85 |
Male |
Co-60 |
15y7m |
Buccal mucosa |
Squamous cell
carcinoma |
Laser treatment |
| 12 |
T2 |
64 |
Female |
Rn-222 |
8y4m |
Tongue |
Squamous cell
carcinoma |
Surgery |
| 13 |
T2 |
78 |
Male |
Rn-222 |
30y11m |
Tongue |
Squamous cell
carcinoma |
Chemotherapy |
| 14 |
T2 |
58 |
Female |
Ra-226 |
11y4m |
Tongue |
Squamous cell
carcinoma |
Surgery |
| 15 |
T2 |
71 |
Male |
Ir-192 |
15y1m |
Tongue |
Squamous cell
carcinoma |
Surgery |
| 16 |
T2 |
61 |
Male |
Ir-192 |
10y5m |
Lower gum |
Squamous cell
carcinoma |
Surgery |
| 17 |
T2 |
76 |
Female |
Ra-226 |
9y8m |
Tongue |
Squamous cell
carcinoma |
Surgery |
| 18 |
T2 |
55 |
Female |
Ra-226 |
17y10m |
Tongue |
Squamous cell
carcinoma |
Surgery |
| 19 |
T2 |
74 |
Female |
Ra-226 |
10y0m |
Tongue |
Squamous cell
carcinoma |
Surgery |
| 20 |
T2 |
74 |
Female |
Ra-226 |
11y9m |
Tongue |
Squamous cell
carcinoma |
Surgery |
| 21 |
T2 |
63 |
Male |
Ra-226 |
11y7m |
Tongue |
Spindle cell carcinoma |
Surgery |
| 22 |
T2 |
58 |
Male |
Ra-226 |
9y0m |
Tongue |
Squamous cell
carcinoma |
Surgery |
| 23 |
T2 |
68 |
Female |
Ra-226 |
8y8m |
Tongue |
Squamous cell
carcinoma |
Surgery |
| 24 |
T2 |
87 |
Female |
Ir-192 |
12y10m |
Tongue |
Squamous cell
carcinoma |
Au-198 |
| 25 |
T2 |
66 |
Male |
Ra-226 |
12y2m |
Tongue |
Squamous cell
carcinoma |
Surgery |
| 26 |
T2 |
76 |
Female |
Ra-226 |
9y5m |
Tongue |
Squamous cell
carcinoma |
Surgery |
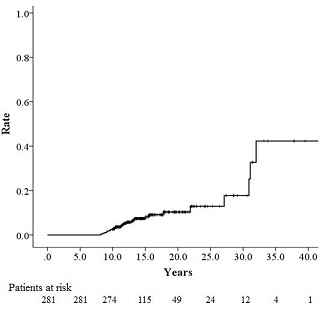
Figure 1: Incidence of second malignancy (SM) in the oral cavity.
Table 2: Relationship between clinical factors and the incidence of second malignancy (SM)
|
Variable |
10-year incidence of SM [%] |
HR |
95%CI |
P value |
|
Sex |
|
|
|
|
|
Male |
0.6 |
1.312 |
0.603-2.853 |
0.304 |
|
Female |
5.3 |
|
UICC
staging and TNM classification |
|
|
|
|
|
Stage I, T1N0 |
1.1 |
1.003 |
0.442-2.278 |
0.735 |
|
Stage II, T2N0 |
3.1 |
|
Age |
|
|
|
|
|
£60 y |
2.1 |
1.944 |
0.812-4.653 |
0.735 |
|
>60
y |
3.4 |
|
Low-dose-rate brachytherapy source
|
|
|
|
|
|
|
Permanent* |
4.5 |
1.272 |
0.476-3.397 |
0.303 |
|
Temporary† |
2.3 |
|
Permanent*:
Au-198, Temporary†: Ir-192,Ra-226,Cs-137,Co-60, Rn-222
Abbreviations:
HR, Hazard Ratio; CI
confidence interval.
|
Twenty patients underwent surgery for SM. Because the other 6 patients had inoperable and/or unresectable lesions, brachytherapy was performed again in 4 patients (Au-198, n = 3; Rn-222, n = 1), chemotherapy was performed in 1 patient, and laser treatment of the buccal mucosa was performed in 1 patient. All 6 patients experienced tumor failure between 4 months and 2 years (median, 1.6 years) after the diagnosis of SM, and 5 of them died of SM between 7 months and 2 years (median, 1.8 years) after the diagnosis of SM. Seventeen (85%) of the 20 patients who were treated with surgery were salvaged and are presently alive. One patient died of neck metastasis at 1.4 years after the diagnosis of SM, 1 patient died of pneumonia at 11.5 years, and 1patient was alive with local failure and neck metastasis.
The 4-year overall survival rate for the 26 patients was 87.8% after the diagnosis of SM. The 4-year overall survival rate (Fig 2) the local control rate (Fig 3) and the disease-free survival rate (Fig 4) for the patients who underwent surgery were 94.4%, 94.7%, and 90%, respectively, and these values were significantly higher than those of the patients who underwent repeat brachytherapy (0%, 25%, and 25%) or other treatments (0% ,0%, and 0%).
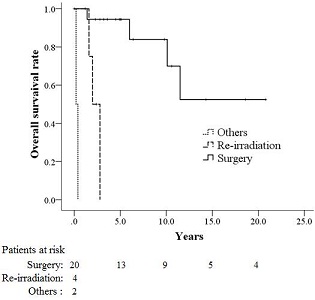
Figure 2: Overall survival rates for patients with second malignancy (SM) according to treatment modality.
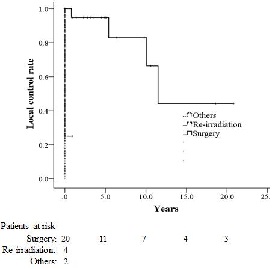
Figure 3: Local control rates for patients with second malignancy (SM) according to treatment modality.
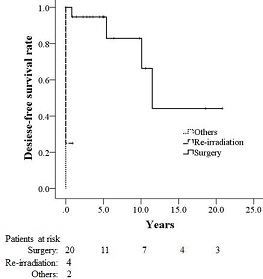
Figure 4: Disease-free survival rates for patients with second malignancy (SM) according to treatment modality.
Although oral ulcers were seen in all the patients after repeat brachytherapy, no severe complications occurred in any of the patients who underwent surgery.
Discussion
There has been no report concerning SM after brachytherapy for tongue cancer, to our knowledge. However some studies have revealed the incidence of SM after brachytherapy for prostate cancer or uterine cancer. Hinnen et al. [8] analyzed 1187 patients after prostate brachytherapy using I-125 and reported the 10-year incidence of SM in the pelvis as a heavily irradiated area: 1.7% in the colon, 1.1% in the rectum, and 1.5% in the bladder. They compared the incidence of SM after brachytherapy and that after prostatectomy and found no difference. However, their analysis also showed that, after brachytherapy, there was an increased risk of bladder SM during the first 4 years after treatment and in patients aged 60 years or younger. Zelefsky et al. [9] also analyzed 413 patients who had received prostate brachytherapy using I-125, and the SM in the pelvis was observed in 1.6% of the patients at 10 years. They compared the incidence with that of 897 patients with intensity-modulated radiotherapy and found no differences in the incidence of SM (IMRT, 4.9%). Ohno et al. [10] investigated the incidence of SM after brachytherapy and external beam radiation therapy (EBRT) for uterine cervical cancer in 2167 patients and reported that 1.8% of them had SM in the pelvis. They estimated the relative risk (RR) by comparing the results with the expected number from population-based cancer registries in Japan and reported that the RRs of leukemia, corpus, bone and soft tissue, and lung cancer were significantly elevated.
The present study showed that SM in the oral cavity after brachytherapy for tongue cancer was observed in 9.2% of the long-term follow-up tongue cancer patients, and the elevated cumulative risk was 17.7% at 30 years after brachytherapy. Although the incidence of SM in our study was higher than in other reports, one of the limitations of our study was that we were unable to confirm a difference between SM and the local recurrence of tongue cancer because the pathology of almost all the SMs in our study was squamous cell carcinoma. However, a previous report has indicated that local recurrence after brachytherapy for tongue cancer always occurs within 5 years [1], and other studies [6,11] have shown that the 10-year local control rate for early-tongue cancer patients treated with brachytherapy was similar to the 5-year local control rate. As the SMs in our study occurred 8 or more years after brachytherapy (median, 11.7 years), the possibility of a local recurrence, rather than SM, is likely to be low. The small number of patients was also a limitation of this study, but the incidence of tongue cancer is lower than those of other malignancies. An additional limitation is that no information on the tobacco and alcohol consumption of the patients was available. Ohno et al. [10] showed the impact of smoking habits on the increased risk of second cancer after cervical cancer treated with radiotherapy, and another report has concluded that tobacco and alcohol consumption are responsible for an estimated 72% of head and neck cancers in the general population [12]. Moreover, it would appear that patients with head and neck cancer continue to have a higher risk of a second cancer even after they stop smoking [13].
The preferable treatment for SM after radiotherapy is surgery, with 5-year survival rates ranging from 16%-36% [14-16]. However, because of the tumor location and extent, medical contraindications, or patient refusal, surgery is often limited and compromised by close or positive margins, and only 20% of patients are able to undergo salvage surgery [14,16]. In this present study, our policy was to perform surgery for patients with operable and resectable tumors and to use repeat brachytherapy for inoperable and/or unresectable cases. The overall survival, local control, and disease-free survival of the patients who underwent surgery were 94.4%, 94.7%, and 90%, respectively, at 4 years, and these values were remarkably superior to those of patients who underwent repeat brachytherapy (0%, 25%, and 25%, respectively). High-dose re-irradiation in inoperable patients is the only treatment option with any potential for a cure. Re-irradiation can be delivered using brachytherapy, stereotactic radiosurgery, intensity-modulated radiotherapy, and so on, with or without chemotherapy and with or without prior debulking surgery. When a SM in a different region other than the treated region occurs, the SM can be treated without considering the toxic effect of re-irradiation [17]. However, for anatomic same-site or sub-adjacent sites of the initial radiotherapy, treatment selection is difficult [18].
Langendijk et al. [19] reported the results of a phase II study of re-irradiation for inoperable and/or unresectable SM (n=26) in the head and neck region. They used EBRT (60-66Gy) to re-irradiate sites in the head and neck region, and the 2-year locoregional control rate was 35%. They observed a late swallowing complication of grade III or IV in 24% of the patients, and nasogastric tube feeding was required in 12% of the patients. However, in our study, all the patients who underwent repeat brachytherapy suffered from radiation ulcers, and none of the cases were salvaged. Moreover, Langendijk et al. [19] concluded that because most recurrences after re-irradiation developed within the boost volume, there are no reasons to use wider margins surrounding the gross-target volume (GTV). Although brachytherapy can be performed for heavy dose irradiation within the GTV and with a small margin, we felt that the definition of the GTV for SM was too difficult to arrange the repeat brachytherapy sources appropriately. There is a possibility that curative radiotherapy for primary cancer may cause slowly developing radiation fibrosis of the irradiated fields over a long term, and such fibrosis may cause insufficient blood flow or a decrease in oxygen at the time of the second radiation therapy.
Conclusion
SM within a prior brachytherapy area in the oral cavity was found after a long interval (≥8 years) in about 1 out of 10 tongue cancer patients treated with brachytherapy, and surgery was a unique salvageable treatment for such cases.
Author’s contribution
EO: performed the literature search and prepared the draft manuscript.
HS: conceived and designed the study and edited the final manuscript.
KN,KA and NH: participated in the study design and helped in the preparation of the manuscript.
All the authors have read and approved the final manuscript.
Funding
No external source of funding.
Acknowledgement
The authors would like to thank all the staff members who participated in this study.
References
[1]. Bourgier C, Coche-Dequeant B, Fournier C, Castelainet B, Prévost B, Lefebvre J, Lartigau E. Exclusive low-doserate brachytherapy in 279 patients with T2N0 mobile tongue carcinoma. Int J Radiat Biol Phys 2005;63:434-440.[Pubmeb]
[2].Kondo M, Hashimoto S, Dokiya T, Inuyama Y, Murakami Y, Nagai T, Asanami S, Fukutake K. Local control of squamous cell carcinoma of the mobile tongue: An experience if different modalities. Int J Radiat Biol Phys 1986;12:755-760.[Pubmeb]
[3].Jegu J, Binder-Foucarda F, Borelc C, Veltena C. Trends over three decades of the risk of second primary cancer among patients with head and neck cancer. Oral Oncol 2013;49:9-14.[Pubmeb]
[4].Farhadieh RD, Otahalb B, Reesc CGG, Salardinid A, Russelle P, Smeef R. Radiotherapy is not associated with an increased rate of second primary tumours in oral squamous carcinoma: a study of 370 patients. Oral Oncol 2009;45:941-945.[Pubmeb]
[5].Yoshimura R, Shibuya H, Hayashi K, Nakagawa K, Toda K, Watanabe H Kaida A, Miura M. Repeat Brachytherapy for Patients With Residual or Recurrent Tumors of Oral Cavity. Int J Radiat Oncol Biol Phys 2012;83:1198-1204.[Pubmeb]
[6].Shibuya H, Hoshina M, Takeda M, Matsumoto S, Suzuki S, Okada N.Brachytherapy for stage I & II oral tongue cancer: An analysis of past cases focusing on control and complications. Int J Radiat Oncol Biol Phys 1993 ;26:51-58.[Pubmeb]
[7].Shibuya H, Takagi M, Kitagawa M, Shioiri S. Squamous cell carcinoma of the oral cavity after irradiation for nonmalignant lesions: Report of four cases. J Oral Maxillofac Surg 1992 ;50:66-71.[Pubmeb]
[8].Hinnen KA, Schaapveld M, van Vulpen M, Battermann JJ, van der Poel H, van Oort IM, van Roermund JG, Monninkhof EM. Prostate brachytherapy and second primary cancer risk: a competitive risk analysis. J Clin Oncol 2011;29:4510-4515.[Pubmeb]
[9].Zelefsky MJ, Housman DM, Pei X, Alicikus Z, Magsanoc JM, Dauer LT, Germain JS, Yamada Y, Kollmeier M, Cox B, Zhang Z. Incidence of secondary cancer development after high-dose intensity-modulated radiotherapy and image-guided brachytherapy for the treatment of localized prostate cancer. Int J Radiat Oncol Biol Phys 2012;83:953-959.
[10].Ohno T, Kato S, Sato S, Fukuhisa K, Nakano T, Tujii H, Arai T. Long-term survival and risk of second cancer after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2007;69:740-745.[Pubmeb]
[11].Kobayashi Y, Karasawa K, Komiya Y, Hanyu N, Okamoto M, Chang TC, Kiguchi Y, Uchida I, Yoshida S, Asoda S. Therapeutic results for 100 patients with cancer of the mobile tongue treated with low dose rate interstitial irradiation. Anticancer res 2007;27:1689-1692.[Pubmeb]
[12].Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, Curado MP, Maso LD, Daudt AW, Fabianova E, Fernandez L, Wünsch-Filho V, Franceschi S, Hayes RB, Herrero B, Kelsey K, Koifman S, Vecchia CL, Lazarus P, Levi F, Lence JJ, Mates D, Matos E, Menezes A, McClean MD, Muscat J, Eluf-Neto J, Olshan AF, Purdue M, Rudnai P, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the international head and neck cancer epidemiology consortium. Cancer Epidermiol Biomarkers Prev 2009;18:541-550.
[13].Leon X, Quer M, Diez S, Orus C, Lopez-Pousa A, Burgues J. Second neoplasm in patients with head and neck cancer. Head & Neck 1999 ;21(3)204-210.[Pubmeb]
[14].Kao J, Garofalo MC, Milano MT, Chmura SJ, Citron JR, Haraf DJ. Reirradiation of recurrent and second primary head and neck malignancies: a comprehensive review. Cancer Treat Rev 2003; 29:21-30.[Pubmeb]
[15].De Crevoisier R, Domenge C, Wibault P, Koscielny S, Lusinchi A, Janot F, Bobin S, Luboinski B, Eschwege F, Bourhis J. Full dose reirradiation combined with chemotherapy after salvage surgery in head and neck carcinoma. Cancer 2001;91:2071-2076.[Pubmeb]
[16].Temam S, Pape E, Janot F, Wibault P, Julieron M, Lusinchi A, Mamelle G, Marandas P, Luboinski B, Bourhis J. Salvage surgery after failure of very accelerated radiotherapy in advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2005;62:1078-83.[Pubmeb]
[17].Shibuya H, Hoshina M, Takeda M, Matsumoto S, Suzuki S, Okada N. Brachytherapy for stage I & II oral tongue cancer: An analysis of past cases focusing on control and complications. Int J Radiat Oncol Biol Phys 1993;26:51-58.[Pubmeb]
[18].Amemiya K, Shibuya H, Yoshimura R, Okada N. The risk of radiation-induced cancer in patients with squamous cell carcinoma of the head and neck and its results of treatment. Br J Radiology 2005;78:1028-33.[Pubmeb]
[19].Langendijk JA, Kasperts N, Leemans CR, Doornaert P, Slotman BJ. A phase II study of primary reirraidiation in squamous cell carcinoma of head and neck. Radiother Oncol 2006;78:306-312.[Pubmeb]

