Research

Steady decrease of the time interval between the occurrence of the primary and subsequent primary early (stage I-II) head and neck cancers
Mio Kojima, Hitoshi Shibuya, Keiji Hayashi, Keiko Yuasa-Nakagawa, Naoki Harata
- Department of Radiation Oncology, Faculty of Medicine, Tokyo Medical and Dental University, Tokyo, Japan
- Submitted: December 28, 2012
- Accepted: January 10, 2013
- Published: January 23, 2013
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
For patients with early-stage (stage I-II) cancers in the head and neck, a serious cause of death is second primary cancers. To improve the survival of these patients, early detection of subsequent new primary cancers is essential. The purpose of this study was to investigate the time-intervals about multiple cancers in the head and neck of early-stage.
Study design
A retrospective study was performed using a database of our institution.
Material and methods
A total of 2,144 patients with early-stage squamous cell carcinoma of the head and neck were reviewed. We calculated the time intervals between the detection of each new primary cancer and the date of diagnosis of the index primary cancer.
Results
249 subsequent primary cancers were documented in 172 patients. The median time-interval between the first cancer and the detection of the second primary cancer was 4.2 years, with steady shortening of the interval: between the second and third primary cancers was 1.7 years, and between the third and fourth primary cancers was 0.9 years. Regardless of the number of subsequent cancers, the second cancers developed in about 4 years after initial treatment.
Conclusions
We demonstrate that the time intervals of the occurrence of subsequent primary cancers steadily shorten along with the increase of the number of new primary early-stage head and neck cancers. This indicates the need for increasing both the frequency and duration of follow-up, so as to ensure early detection of subsequent new primary cancers.
Keywords
second primary cancer, multiple cancers, time interval, head and neck, early stage.
Introduction
Head and neck cancers account for about 5% of all cancers in Japan [1], and for 8 and 3% of the cancer deaths in men and women, respectively [2]. While advances in the therapeutic modalities, including surgery, radiotherapy and chemotherapy, have led to improvements of local growth of squamous cell carcinoma of the head and neck [3], they do not appear to have had any remarkable influence on the survival of these patients [4]. In patients with early-stage (stages I and II) head and neck cancer, the major factor affecting the survival rate is cervical lymph node metastasis [5,6], followed by the frequency of the development of second primary cancers [7]. Treatment of a second primary cancer is often less successful than that of the index primary cancer [8,9], due to the late diagnosis and /or difficulty in administering aggressive therapies owing to the presence of the complications from the previous cancer treatment [10]. The location of the second primary cancer also plays a major role in the outcome. In this connection, patients who developed second primary cancers in the esophagus or lung have a higher mortality rate than those who developed second primaries at other sites [10].
Patients with head and neck cancers are known to be at an increased risk of developing of second primary cancers again in the aero digestive tract [11-13], with the incidence of a second primary cancer in head and neck region being reported to be approximately 3-4% per year [4, 7, 14]. As one of the reasons for this elevated risk, the “field cancerization” theory has been proposed [15]. This theory suggests that when a wide field of epithelium that is preconditioned to develop cancer is repeatedly exposed to carcinogens, multiple, independent malignant lesions can develop in the exposed epithelium. In the head and neck region, cigarette smoking and alcohol consumption are thought to be a major risk factor [16-18]. The other reasons include age at diagnosis of the index primary cancer, the treatment for it, HPV infection, and genetics.
Furthermore, some patients develop multiple primary cancers, so that it is essential to carefully follow-up the progress of patients with head and neck cancer and detect subsequent new primary cancers as early as possible to improve the survival of these patients. To our knowledge, however, no studies have been conducted to investigate the differences in the time to the development of each primary cancer in head and neck cancer patients with successively occurring new primary cancers. Thus, to address this issue, we conducted an investigation of the time intervals to the development of each of successive primary cancer at the site of the oral cavity and oropharynx.
A brief statement: We demonstrate here that the time intervals of the occurrence of subsequent primary cancers steadily shorten along with the increase of the number of new primary head and neck cancers.
Material and methods
A retrospective review was conducted using a database established by data collected continuously from 1956 to 2010 at our institution. This study was given the approval of the Institutional Ethics Committee of Tokyo Medical and Dental University Hospital and had followed the guidelines of the Helsinki Declaration. The database included 2,144 patients with stage I-II squamous cell carcinoma of the head and neck. The site of the primary cancer in this population was the tongue in 1,225 patients, floor of the mouth in 234 patients, buccal mucosa in 189 patients, upper gum in 146 patients, lower gum in 85 patients, lip in 23 patients, and oropharynx in 242 patients. Our data review included the age and gender of the subjects, the treatments administered, and the date of diagnosis and treatment of the index and subsequent primary cancers. From these data, we calculated the time intervals between the detection of each new primary cancer and the date of diagnosis of the preceding primary cancer. The treatment modalities were brachytherapy, external radiation, surgery, chemotherapy, and laser therapy, with or without external radiation. Some of the treatments, except for brachytherapy, had been undertaken at other hospitals in some cases. At our institute, patients are examined every 2 weeks during the first 3 months after the initial treatment, every 3 months for at least the subsequent 3 years, and every half or one year thereafter, with the patients advised to contact the hospital at any time in case of need.
For the diagnosis of multiple cancers, the criteria utilized in this study were those suggested by the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute [19], and also those by Warren and Gates [20] and modified by Hong et al. [21]. According to these criteria, multiple primary lesions with the same histology arising in different parts of the same organ are considered as separate primary cancers; a second primary cancer has to be separated from the first by at least 2cm of normal epithelium. Only cases with stage I-II squamous cell carcinoma were included for the analysis in this study. Cases whose medical records defined the new cancer as recurrent, metastatic or radiation-induced were not included in this study. The subsites of occurrence of the subsequent primary cancers of the head and neck included the tongue, floor of the mouth, buccal mucosa, upper gum, lower gum, lip, and oropharynx.
Statistical analysis was conducted using the SPSS program. Since the data in respect of the intervals were verified not to show normal distribution by a P-P plot, the Mann-Whitney U test, a non-parametric statistical test, was used to determine the statistical significance of the differences in the intervals. P values of less than 0.05 were considered as being indicative of statistical significance.
Result
A total of 249 subsequent primary cancers in 172 patients were included in the analysis. The percentage of patients with incidence of subsequent primary cancers in the head and neck was 8.0% (172/2,144 patients). The patients consisted of 105 males (61%) and 67 females (39%), with a mean age of the patients at the time of treatment at our institute of 66.3 years (range, 21 to 85 years). The shortest follow-up duration was 4 months, while the longest was 36 years. Of the 172 patients, 116 patients had only one subsequent primary cancer, 35 patients had two, 21 patients had three (Table 1). The site distribution of the first primary cancers in the 172 patients was composed of 71 (41.3%) tongue, 22 (12.8%) floor of mouth, 20 (11.6%) buccal mucosa, 12 (7.0%) upper gum, 27 (15.7%) lower gum , 17 (9.9%) oropharynx, 3 (1.7%) lip.
Table 1: Patients with the number of subsequent primary cancers
|
No. (%) |
Incidence
(n = 2144)
|
| Patients with 1 SPC |
116 (67.5) |
5.4% |
| Patients with 2 SPCs |
35 (20.3) |
1.6% |
| Patients with 3 SPCs |
21 (12.2) |
1.0% |
| Total |
172 (100) |
8.0% |
Table 2: Distribution of the subsequent primary cancers
| Site |
No. of primary cancers |
No. (%) |
|
Second |
Third |
Fourth |
Total |
| Tongue |
42 |
17 |
3 |
62 (24.9) |
| Floor of mouth |
13 |
0 |
0 |
13(5.2) |
| Buccal mucosa |
33 |
10 |
8 |
51 (20.5) |
| Upper gum |
9 |
3 |
2 |
14 (5.6) |
| Lower gum |
23 |
7 |
2 |
32 (12.9) |
| Lip |
3 |
3 |
1 |
7 (2.8) |
| Oropharynx |
49 |
16 |
5 |
70 (28.1) |
| Total |
172 |
56 |
21 |
249 (100) |
Table 2 shows the site distribution of the subsequent primary cancers. The majority developed in the oropharynx (70/249 cases, 28.1%), followed in frequency by the tongue (62/249 cases, 24.9%) and buccal mucosa (51/249 cases, 20.5%). About a half (89/172, 51.7%) of the patients were treated for their first cancers by brachytherapy with or without external radiation, and 62 of the 172 (36.0%) patients were treated by surgery.
Table 3: Time intervals between the occurrence of the index primary cancers and the subsequent primary cancers
|
Time intervals |
| Period |
Median |
Mean |
Min |
Max |
| Between the first and second cancers |
4.2 years |
6.0 years |
0 month |
35.0 years |
| Between the second and third cancers |
1.7 years |
3.3 years |
1.2 months |
15.5 years |
| Between the third and fourth cancers |
0.9 years |
0.9 years |
1.2 months |
7.6 years |
The median time intervals to the occurrence of the subsequent primary cancer were as follows: between the first cancer and second primary cancer, 4.2 years (min 0 month, max 35.1 years, mean 5.8 years); between the second and third primary cancers, 1.7 years (min 1.2 months, max 15.5 years, mean 3.3 years); between the third and fourth primary cancers, 0.9 years (min 1.2 months, max 7.6 years, mean 1.8 years) (Table 3). Significant differences were noted in the time intervals from the occurrence of the first primary cancer to that of the second primary cancer and the time interval from the occurrence of the second primary cancer to that of the third primary cancer (p = 0.004). Similarly, significant differences were also noted in the time intervals from the occurrence of the second primary cancer to that of the third primary cancers and the time interval from the occurrence of the third primary cancer to that of the fourth primary cancer (p = 0.038). The results of the statistical analysis indicated that the time intervals between the index cancer and the occurrence of the subsequent primary cancer shortened steadily with the increase in the number of new primary cancers in each patient (Figure 1).
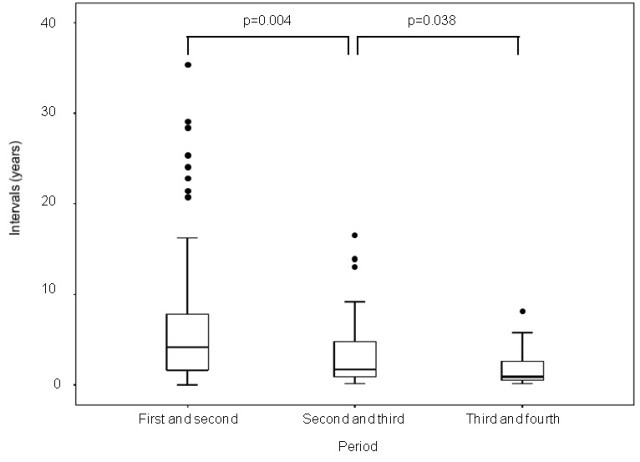
Figure 1: Shortened interval between primary and subsequent primary cancers
The median time interval between the first and second primary cancers in patients with only one subsequent primary cancer (n = 116) was 3.9 years (mean 5.7 years) and that in patients with more than two subsequent cancers (n = 56) was 4.4 years (mean 6.1 years). No significant difference in the time interval between the first and second primary cancers was noted between these two groups (p = 0.171) (Table 4). Thus, irrespective of the number of subsequent primary cancers, the time interval between the occurrence of the first and second primary cancers remained unchanged.
Table 4: Time intervals from the occurrence of the first to the second primary cancers by the number of subsequent primary cancers
|
Only 1 subsequent
cancer |
More than 2 subsequent
cancers |
|
|
n = 116 (67.4%) |
n = 56 (32.6%) |
|
| Median (years) |
3.9 |
4.4 |
p=0.171 |
| Mean (years) |
5.7 |
6.1 |
|
No gender difference was noted in any of the time intervals : from the occurrence of the first to that of the second primary cancer (median 4.2 years (male, n = 104), 4.0 years (female, n = 63), p = 0.304); from the occurrence of the second to that of the third primary cancer (median 1.5 years (male, n = 24), 2.1 years (female, n = 32), p = 0.471); from the occurrence of the third to that of the fourth primary cancer (median 0.7 years (male, n = 9), 1.2 years (female, n = 12), p = 0.303).
After undergoing radiotherapy for head and neck cancer, 92 of the 2,144 (4.3%) patients developed subsequent primary cancers in the esophagus and 74 (3.5%) developed subsequent primary cancers in the lung. The median time to the detection of the second primary cancer was 2.0 years for the esophagus (range, 0-24 years) and 5.4 years for the lung (range, 0-31.5 years).
Discussion
In our study, the median time interval to the development of a second primary cancer from the diagnosis of the first primary cancer in the head and neck was 4.2 years, consistent with the findings of previous studies, in which the interval ranged from around 3 to 4 years [4, 8, 10]. Regardless of the number of subsequent primary cancers, the second primary cancer developed a median of about 4 years after the treatment of the first cancer in our study; thereafter, the time intervals from the index primary cancer to the subsequently developing new primary cancer decreased; second to third, 1.7 years; third to fourth, 0.9 years. Thus, our present study revealed that the time intervals to the occurrence of the subsequent primary cancer from the index cancer decreased steadily with each successively developing new primary cancer in each patient. To the best of our knowledge, this is the first statistical demonstration regarding the decrease of time intervals between index primary and subsequent second primary cancers. Although the locoregional control rate of early-stage primary head and neck cancers is quite good, these patients often die from the subsequent development of new primary cancer rather than from recurrence of the index primary cancer itself. Our findings emphasize the need for increasing both the frequency and duration of follow-up of head and neck cancer patients who have already developed a second new primary cancer, so as to ensure early detection of subsequent new primary cancers.
The reason for the steadily decreasing time intervals between successively occurring new primary cancers remains unclear. Based on previous literature, each or all of the “field cancerization” theory, common risk factors such as tobacco and alcohol consumption, p53 expression, HPV infection, and genetics may likely play roles. Further studies are necessary to investigate any relevance of these factors to the time-course of multiple cancers.
In regard to the site specification, it is known that if the first cancer develops in the oral cavity and oropharynx, the most frequent site of development of the second cancer is also the oral cavity and oropharynx [12, 13]. The anatomical closeness of the successive primary cancer sites may be the reason for the earlier detection of subsequent primary cancers of the head and neck during follow-up examinations [11]. Because the analysis in this study was restricted to cancers of the head and neck region, there was a possibility that the site specification took a major role to detect new primary cancers earlier.
Since subsequent primary cancers after the diagnosis of primary head and neck cancers are also frequently found in the esophagus and lung [10-12], we examined the time intervals to the development of the subsequent cancers in the esophagus and lung from the initial diagnosis of primary head and neck cancers, but as reference results. The median time to the detection of the second cancer was 2.0 years for the esophagus and 5.4 years for the lung. Our results were close to those of the recent study published by Chen et al., [10].
Conclusions
Our study demonstrates that in patients with early-stage head and neck cancer, the time intervals to the occurrence of a subsequent primary cancer from the index cancer decreased steadily with the development of each successive new primary cancer. The median time intervals from the index primary cancer diagnosis to the occurrence of the subsequent primary cancer were as follows: first to second, 4.2 years; second to third, 1.7 years; third to fourth, 0.9 years. These findings underscore the need for increasing the frequency as well as duration of follow-up of patients with early-stage head and neck cancer, especially those who have already developed a new primary cancer, because early detection of subsequent new primary cancers may lead to improve the survival rate. The benefits of this measure would be expected to be most pronounced in patients with early-stage head and neck cancer.
Author’s contribution
MK: carried out the literature search and prepared the draft manuscript.
HS: conceived and designed the study and edited the final manuscript.
KH, KN, NH: participated in design and helped in preparation of manuscript.
All authors read and approved the final manuscript for submission.
Acknowledgements
None
References
[1]. Matsuda T, Marugame T, Kamo KI, Katanoda K, Ajiki W, Sobue T and The Japan Cancer Surveillance Research Group. Cancer Incidence and Incidence Rates in Japan in 2006: Based on Data from 15 Population-based Cancer Registries in the Monitoring of Cancer Incidence in Japan (MCIJ) Project. Jpn J Clin Oncol 2012; 42:139-147. [Pubmed].
[2]. Center for Cancer Control and Information Services, National Cancer Center, Japan. Vital Statistics Japan (Ministry of Health, Labour and Welfare).c-2012 - [cited 2012 April 15]. Available from: http://ganjoho.jp/professional/statistics/statistics.html
[3]. Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med 1993;328:184-194. [Pubmed].
[4]. Rennemo E, Zatterstrom U, Boysen M. Impact of Second Primary Tumors on Survival in Head and Neck Cancer: An Analysis of 2,063 Cases. Laryngoscope 2008 118:1350-1356. [Pubmed].
[5]. Nakagawa T, Shibuya H, Yoshimura R, Miura M, Okada N, Kishimoto S, Amagasa M, Omura K. Neck node metastasis after successful brachytherapy for early stage tongue carcinoma. Radiother Oncol 2003;68:129-135. [Pubmed].
[6]. Umeda M, Komatsubara H, Ojima Y, Minamikawa T, Shibuya Y, Yokoo S, Ishii J, Komori T. A comparison of brachytherapy and surgery for the treatment of stage I-II squamous cell carcinoma of the tongue. Int J Oral Maxillofac Surg 2005;34:739-744. [Pubmed].
[7]. Yamamoto E, Shibuya H, Yoshimura R, Miura M. Site specific dependency of second primary cancer in early stage head and neck squamous cell carcinoma. Cancer 2002;94:2007-2014. [Pubmed].
[8]. Jones AS, Morar P, Phillips DE, Field JK, Husband D, Helliwell TR. Second Primary Tumors in Patients with Head and Neck Squamous Cell Carcinoma. Cancer 1995;75:1343-1353. [Pubmed].
[9]. Tsou YA, Hua CH, Tseng HC, Lin MH, Tsai MH. Survival study and treatment strategy for second primary malignancies in patients with head and neck squamous cell carcinoma and nasopharyngeal carcinoma. Acta Oto-Laryngol 2007;127:651-657. [Pubmed].
[10]. Chen MC, Huang WC, Chan CH, Chen PT, Lee KD. Impact of second primary esophageal or lung cancer on survival of patients with head and neck cancer. Oral Oncol 2010;46:249-254. [Pubmed].
[11]. Chuang SC, Scelo G, Tonita JM, Tamaro S, Jonasson JG, Kliewer EV, Hemminki K, Weiderpass E, Pukkala E, Tracey E, Friis S, Kirn VP, Brewster DH, Martos C, Chia KS, Boffetta P, Brennan P, Hashibe M. Risk of second primary cancer among patients with head and neck cancers: A pooled analysis of 13 cancer registries. Int J Cancer 2008;123:2390-2396. [Pubmed].
[12]. Morris LG, Sikora AG, Hayes RB, Patel SG, Ganly I. Anatomic sites at elevated risk of second primary cancer after an index head and neck cancer. Cancer Cause Control 2011;22:671-679. [Pubmed].
[13]. Leon X, Quer M, Diez S, Orus C, Pousa AL, Burgues J. Second neoplasm in patients with head and neck cancer. Head Neck 1999;21:204-210. [Pubmed].
[14]. Day GL, Blot WJ. Second primary tumors in patients with oral cancer. Cancer 1992;70:14-19. [Pubmed].
[15]. Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. Cancer 1953;6:963-968. [Pubmed].
[16]. Maier H, Dietz A, Gewelke U, Heller WD, Weidauer H. Tobacco and alcohol and the risk of head and neck cancer. Clin Investig 1992;70:320-327. [Pubmed].
[17]. Do KA, Johnson MM, Lee JJ, Wu XF, Dong Q, Hong WK, Khuri FR, Spits MR. Longitudinal study of smoking patterns in relation to the development of smoking-related secondary primary tumors in patients with upper aerodigestive tract malignancies. Cancer 2004;101:2837-2842. [Pubmed].
[18]. Freedman ND, Abnet CC, Leitzmann MF, Hollenbeck AR, Schatzkin A. Prospective investigation of the cigarette smoking-head and neck cancer association by sex. Cancer 2007;110:1593-1601. [Pubmed].
[19]. Johnson CH, Peace S, Adamo P, Fritz A, Percy-Laurry A, Edwards BK. The 2007 Multiple Primary and Histology Coding Rules. National Cancer Institute, Surveillance, Epidemiology and End Results Program. Bethesda, MD, 2007.
[20]. Warren S, Gates DC. Multiple primary malignant tumors: a survey of the literature. Am J Cancer 1932;16:1358-1414.
[21]. Hong HK, Lippman SC, Itri L, Karp DD, Lee JS, Byers RM, Schantz SP, Kramer AM, Lotan R, Peters LJ, Dimery IW, Brown BW, Goepfert H. Prevention of second primary tumors with isotretinoin in squamous cell carcinoma of the head and neck. N Engl J Med 1990;323:795-801. [Pubmed].

